Abstract
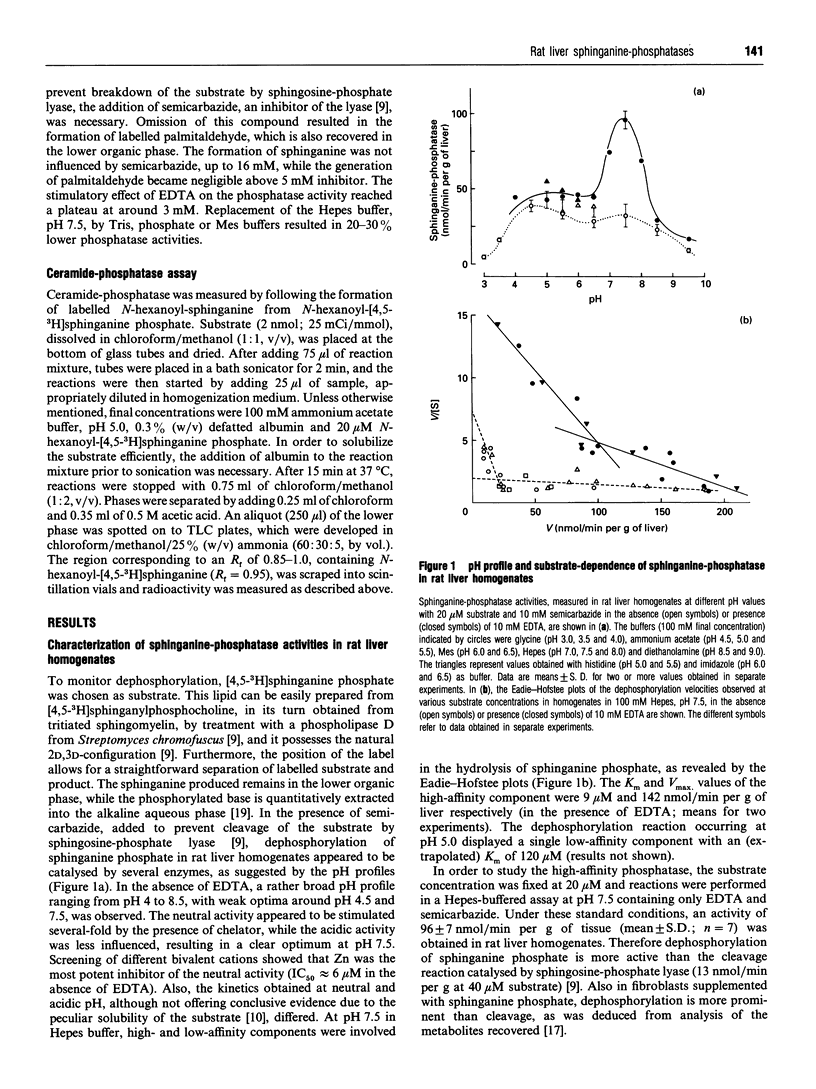
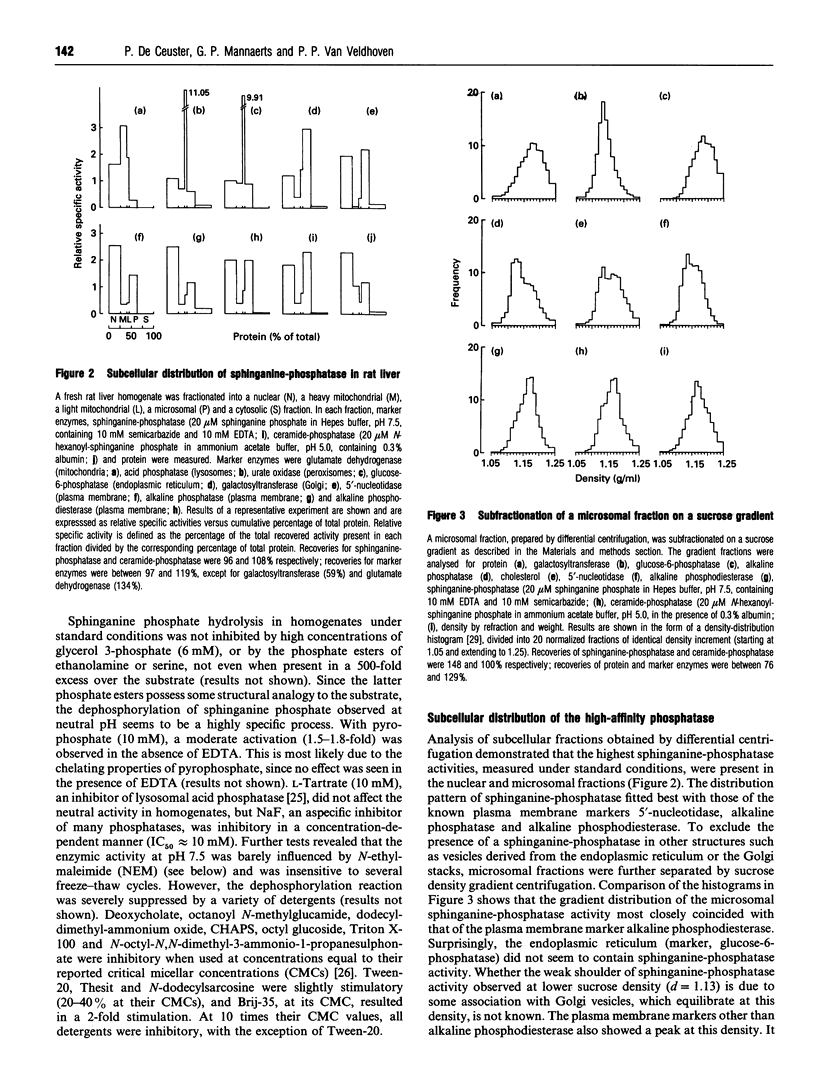
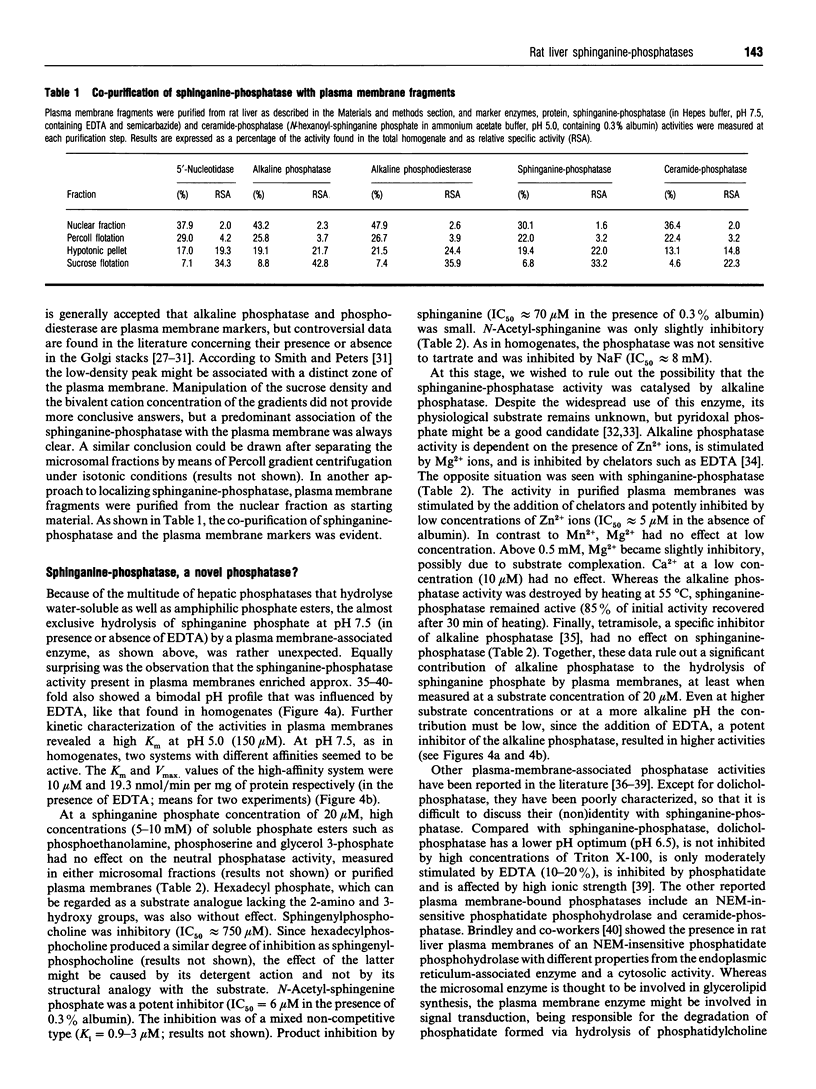
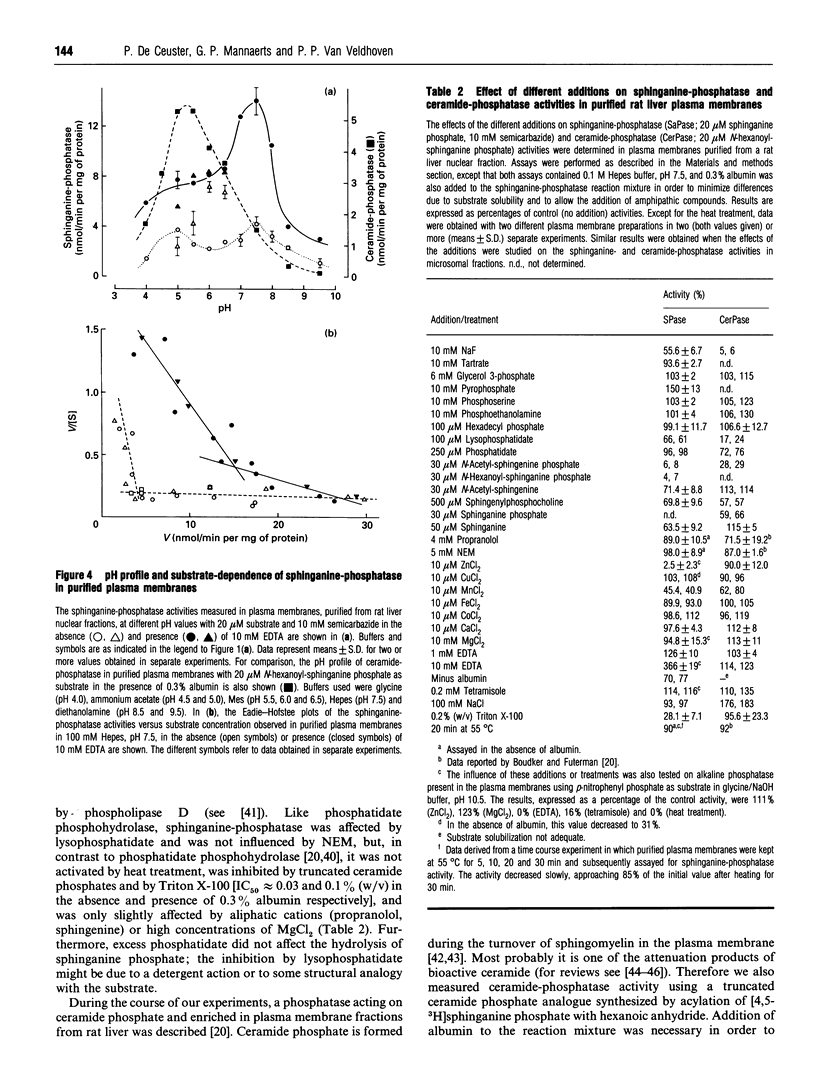
One of the primary products of [4,5-3H]sphinganine phosphate, added to fibroblast cultures, is sphinganine [Van Veldhoven and Mannaerts (1994) Biochem. J. 299, 597-601], implicating the physiological action of (a) hitherto unknown phosphatase(s). We have now further characterized this activity in rat liver. In homogenates, the dephosphorylation appeared to be catalysed by multiple enzymes. A low-affinity system was active at acidic pH, whereas at physiological pH values hydrolysis was carried out by a high-affinity enzyme. The latter was sensitive to Zn2+ and detergents and possessed a pH optimum of 7.5. Upon cell fractionation the major portion of the high-affinity activity was recovered in the nuclear and microsomal fractions. Further separation of the microsomal fraction showed an association predominantly with vesicles derived from the plasma membrane. Likewise, when plasma membranes were prepared from the nuclear fraction, the high-affinity phosphatase co-purified with the plasma membrane markers. From the differential effects of bivalent cations, chelators, water-soluble and amphiphilic phosphate esters, detergents and other compounds, it could be concluded that the plasma membrane-associated sphinganine-phosphatase activity is not due to alkaline phosphatase, dolichol-phosphatase, the N-ethylmaleimide-insensitive phosphatidate phosphatase or ceramide-phosphatase. The dephosphorylation observed at acidic pH in homogenates appeared also to be enriched in purified plasma membranes and might represent a side-activity of ceramide-phosphatase. We speculate that the high-affinity phosphatase, which is especially active in neuronal tissues, plays a role in the attenuation of bioactive phosphorylated sphingoid bases such as sphingenine phosphate, and propose to name it sphingosine-phosphatase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar-Costesec A., Wibo M., Thinès-Sempoux D., Beaufay H., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. IV. Biochemical, physical, and morphological modifications of microsomal components induced by digitonin, EDTA, and pyrophosphate. J Cell Biol. 1974 Sep;62(3):717–745. doi: 10.1083/jcb.62.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. 3. Subfractionation of the microsomal fraction by isopycnic and differential centrifugation in density gradients. J Cell Biol. 1974 Apr;61(1):213–231. doi: 10.1083/jcb.61.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudker O., Futerman A. H. Detection and characterization of ceramide-1-phosphate phosphatase activity in rat liver plasma membrane. J Biol Chem. 1993 Oct 15;268(29):22150–22155. [PubMed] [Google Scholar]
- Brightwell R., Tappel A. L. Lysosomal acid pyrophosphatase and acid phosphatase. Arch Biochem Biophys. 1968 Mar 20;124(1):333–343. doi: 10.1016/0003-9861(68)90335-4. [DOI] [PubMed] [Google Scholar]
- Buehrer B. M., Bell R. M. Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. J Biol Chem. 1992 Feb 15;267(5):3154–3159. [PubMed] [Google Scholar]
- Buehrer B. M., Bell R. M. Sphingosine kinase: properties and cellular functions. Adv Lipid Res. 1993;26:59–67. [PubMed] [Google Scholar]
- Desai N. N., Zhang H., Olivera A., Mattie M. E., Spiegel S. Sphingosine-1-phosphate, a metabolite of sphingosine, increases phosphatidic acid levels by phospholipase D activation. J Biol Chem. 1992 Nov 15;267(32):23122–23128. [PubMed] [Google Scholar]
- Dressler K. A., Kolesnick R. N. Ceramide 1-phosphate, a novel phospholipid in human leukemia (HL-60) cells. Synthesis via ceramide from sphingomyelin. J Biol Chem. 1990 Sep 5;265(25):14917–14921. [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Farquhar M. G., Bergeron J. J., Palade G. E. Cytochemistry of Golgi fractions prepared from rat liver. J Cell Biol. 1974 Jan;60(1):8–25. doi: 10.1083/jcb.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedde K. N., Whyte M. P. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridoxal-5'-phosphate ectophosphatase: normal and hypophosphatasia fibroblast study. Am J Hum Genet. 1990 Nov;47(5):767–775. [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J., Gill D. L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990 Jun 29;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Linardic C. M. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993 Dec 21;1154(3-4):223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Hirschberg C. B., Kisic A., Schroepfer G. J., Jr Enzymatic formation of dihydrosphingosine l-phosphate. J Biol Chem. 1970 Jun;245(12):3084–3090. [PubMed] [Google Scholar]
- Jamal Z., Martin A., Gomez-Muñoz A., Brindley D. N. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J Biol Chem. 1991 Feb 15;266(5):2988–2996. [PubMed] [Google Scholar]
- Keenan R. W., Haegelin B. The enzymatic phosphorylation of sphinganine. Biochem Biophys Res Commun. 1969 Dec 4;37(6):888–894. doi: 10.1016/0006-291x(69)90214-9. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N., Hemer M. R. Characterization of a ceramide kinase activity from human leukemia (HL-60) cells. Separation from diacylglycerol kinase activity. J Biol Chem. 1990 Nov 5;265(31):18803–18808. [PubMed] [Google Scholar]
- Louie D. D., Kisic A., Schroefer G. J., Jr Sphingolipid base metabolism. Partial purification and properties of sphinganine kinase of brain. J Biol Chem. 1976 Aug 10;251(15):4557–4564. [PubMed] [Google Scholar]
- Mathias S., Kolesnick R. Ceramide: a novel second messenger. Adv Lipid Res. 1993;25:65–90. [PubMed] [Google Scholar]
- Mattie M., Brooker G., Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem. 1994 Feb 4;269(5):3181–3188. [PubMed] [Google Scholar]
- Neugebauer J. M. Detergents: an overview. Methods Enzymol. 1990;182:239–253. doi: 10.1016/0076-6879(90)82020-3. [DOI] [PubMed] [Google Scholar]
- Olivera A., Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993 Oct 7;365(6446):557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Sadahira Y., Ruan F., Hakomori S., Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9686–9690. doi: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadahira Y., Zheng M., Ruan F., Hakomori S., Igarashi Y. Sphingosine-1-phosphate inhibits extracellular matrix protein-induced haptotactic motility but not adhesion of B16 mouse melanoma cells. FEBS Lett. 1994 Feb 28;340(1-2):99–103. doi: 10.1016/0014-5793(94)80180-0. [DOI] [PubMed] [Google Scholar]
- Sarrouilhe D., Lalegerie P., Baudry M. Alkaline phosphatase activity at physiological pH: kinetic properties and biological significance. Cell Mol Biol (Noisy-le-grand) 1993 Feb;39(1):13–19. [PubMed] [Google Scholar]
- Smith G. D., Peters T. J. Analytical subcellular fractionation of rat liver with special reference to the localisation of putative plasma membrane marker enzymes. Eur J Biochem. 1980 Feb;104(1):305–311. doi: 10.1111/j.1432-1033.1980.tb04429.x. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Hellenbroich B., Heimann G. Properties and specificities of sphingosine kinase from blood platelets. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1311–1316. doi: 10.1515/bchm2.1973.354.2.1311. [DOI] [PubMed] [Google Scholar]
- Strout H. V., Vicario P. P., Saperstein R., Slater E. E. A protein phosphotyrosine phosphatase distinct from alkaline phosphatase with activity against the insulin receptor. Biochem Biophys Res Commun. 1988 Mar 15;151(2):633–640. doi: 10.1016/s0006-291x(88)80328-0. [DOI] [PubMed] [Google Scholar]
- Van Belle H. Kinetics and inhibition of alkaline phosphatases from canine tissues. Biochim Biophys Acta. 1972 Nov 10;289(1):158–168. doi: 10.1016/0005-2744(72)90118-0. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Brees C., Mannaerts G. P. D-aspartate oxidase, a peroxisomal enzyme in liver of rat and man. Biochim Biophys Acta. 1991 Jan 23;1073(1):203–208. doi: 10.1016/0304-4165(91)90203-s. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., De Ceuster P., Rozenberg R., Mannaerts G. P., de Hoffmann E. On the presence of phosphorylated sphingoid bases in rat tissues. A mass-spectrometric approach. FEBS Lett. 1994 Aug 15;350(1):91–95. doi: 10.1016/0014-5793(94)00739-x. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Foglesong R. J., Bell R. M. A facile enzymatic synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. J Lipid Res. 1989 Apr;30(4):611–616. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Sphinganine 1-phosphate metabolism in cultured skin fibroblasts: evidence for the existence of a sphingosine phosphatase. Biochem J. 1994 May 1;299(Pt 3):597–601. doi: 10.1042/bj2990597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver. J Biol Chem. 1991 Jul 5;266(19):12502–12507. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Matthews T. J., Bolognesi D. P., Bell R. M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem Biophys Res Commun. 1992 Aug 31;187(1):209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- Yokota S., Himeno M., Kato K. Immunocytochemical localization of acid phosphatase in rat liver. Cell Struct Funct. 1989 Apr;14(2):163–171. doi: 10.1247/csf.14.163. [DOI] [PubMed] [Google Scholar]
- Zhang H., Desai N. N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991 Jul;114(1):155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veldhoven P. P., Mannaerts G. P. Sphingosine-phosphate lyase. Adv Lipid Res. 1993;26:69–98. [PubMed] [Google Scholar]


