Abstract
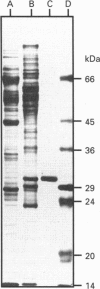
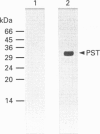
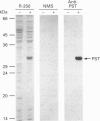
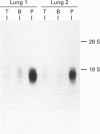
Phenol sulphotransferases esterify both endogenous and foreign hydroxylated aromatic compounds with sulphate. Since these enzymes participate in both hormone and drug metabolism, elucidating their regulation at both the enzymic and molecular levels may provide new understanding in several metabolic pathways. The primary structure of a bovine phenol sulphotransferase has been determined by isolation of the corresponding cDNA. Two partial bovine cDNAs were first isolated by probing a tracheal epithelial cell lambda gt11 cDNA library with a rat phenol sulphotransferase cDNA. These clones provided the sequences of the 5' and 3' ends of the predicted coding region. A contiguous cDNA was subsequently isolated by PCR using 5' and 3' oligonucleotide primers and the cDNA library as the template. The sequence of the resulting approx. 1 kbp cDNA predicted an amino acid sequence that included sequences determined for several tryptic peptides of the purified protein. Antiserum directed to a synthetic N-terminal peptide predicted by the cDNA sequence showed reactivity with the purified enzyme. High-level Trc-promoter-driven expression of the recombinant bovine enzyme was achieved in Escherichia coli. The bovine cDNA was used to determine relative steady-state levels of phenol sulphotransferase transcripts in bovine lung tissues; distal lung parenchymal RNA levels were 6-10-fold greater than those in tracheobronchial epithelium. Using a bronchial epithelial cell culture model, however, cortisol was observed to increase mRNA levels by 5-fold in both a dose- and time-dependent manner; this corresponds to previously reported glucocorticoid stimulation of phenol sulphotransferase activity in this system [Beckmann, Illig and Bartzatt (1994) J. Cell Physiol. 160, 603-610].
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy I. A., Wood T. C., Weinshilboum R. Human liver estrogen sulfotransferase: identification by cDNA cloning and expression. Biochem Biophys Res Commun. 1994 May 16;200(3):1621–1629. doi: 10.1006/bbrc.1994.1637. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Jackson B. L. Human platelet phenol sulfotransferase: stability of two forms of the enzyme with time and presence of a racial difference. Clin Chim Acta. 1984 Apr 13;138(2):185–196. doi: 10.1016/0009-8981(84)90233-x. [DOI] [PubMed] [Google Scholar]
- Autrup H., Wefald F. C., Jeffrey A. M., Tate H., Schwartz R. D., Trump B. F., Harris C. C. Metabolism of benzo[a]pyrene by cultured tracheobronchial tissues from mice, rats, hamsters, bovines and humans. Int J Cancer. 1980 Feb 15;25(2):293–300. doi: 10.1002/ijc.2910250219. [DOI] [PubMed] [Google Scholar]
- Barańczyk-Kuźma A. Phenol sulfotransferase in human lung. Biochem Med Metab Biol. 1986 Feb;35(1):18–30. doi: 10.1016/0885-4505(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Barańczyk-Kuźma A., Szymczyk T. Extrahepatic sulfation of phenols. Bovine lung and intestinal phenol sulfotransferases. Biochem Pharmacol. 1987 Oct 1;36(19):3141–3146. doi: 10.1016/0006-2952(87)90624-1. [DOI] [PubMed] [Google Scholar]
- Barańczyk-Kuźma A., Szymczyk T. Lung phenol sulfotransferases. Thermal stability of human and bovine enzymes. Biochem Pharmacol. 1986 Mar 15;35(6):995–999. doi: 10.1016/0006-2952(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Bartzatt R., Beckmann J. D. Inhibition of phenol sulfotransferase by pyridoxal phosphate. Biochem Pharmacol. 1994 Jun 1;47(11):2087–2095. doi: 10.1016/0006-2952(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Beckmann J. D. Continuous fluorometric assay of phenol sulfotransferase. Anal Biochem. 1991 Sep 2;197(2):408–411. doi: 10.1016/0003-2697(91)90412-m. [DOI] [PubMed] [Google Scholar]
- Beckmann J. D., Illig M., Bartzatt R. Regulation of phenol sulfotransferase expression in cultured bovine bronchial epithelial cells by hydrocortisone. J Cell Physiol. 1994 Sep;160(3):603–610. doi: 10.1002/jcp.1041600324. [DOI] [PubMed] [Google Scholar]
- Beckmann J. D., Spurzem J. R., Rennard S. I. Phenol sulfotransferase expression in the airways: enzymological and immunohistochemical demonstration. Cell Tissue Res. 1993 Dec;274(3):475–485. doi: 10.1007/BF00314544. [DOI] [PubMed] [Google Scholar]
- Beckmann J. D., Takizawa H., Romberger D., Illig M., Claassen L., Rickard K., Rennard S. I. Serum-free culture of fractionated bovine bronchial epithelial cells. In Vitro Cell Dev Biol. 1992 Jan;28A(1):39–46. doi: 10.1007/BF02631078. [DOI] [PubMed] [Google Scholar]
- Boström H., Wengle B. Studies on ester sulphates. 23. Distribution on phenol and steroid sulphokinase in adult human tissues. Acta Endocrinol (Copenh) 1967 Dec;56(4):691–704. [PubMed] [Google Scholar]
- Buhl A. E., Waldon D. J., Baker C. A., Johnson G. A. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J Invest Dermatol. 1990 Nov;95(5):553–557. doi: 10.1111/1523-1747.ep12504905. [DOI] [PubMed] [Google Scholar]
- Campbell N. R., Van Loon J. A., Sundaram R. S., Ames M. M., Hansch C., Weinshilboum R. Human and rat liver phenol sulfotransferase: structure-activity relationships for phenolic substrates. Mol Pharmacol. 1987 Dec;32(6):813–819. [PubMed] [Google Scholar]
- Cassidy M. K., Houston J. B. Phenol conjugation by lung in vivo. Biochem Pharmacol. 1980 Feb;29(3):471–474. doi: 10.1016/0006-2952(80)90535-3. [DOI] [PubMed] [Google Scholar]
- Choli T., Kapp U., Wittmann-Liebold B. Blotting of proteins onto Immobilon membranes. In situ characterization and comparison with high-performance liquid chromatography. J Chromatogr. 1989 Aug 4;476:59–72. doi: 10.1016/s0021-9673(01)93856-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dalhoff K., Poulsen H. E. Simultaneous measurements of glutathione and activated sulphate (PAPS) synthesis rates and the effects of selective inhibition of glutathione conjugation or sulphation of acetaminophen. Biochem Pharmacol. 1993 Aug 3;46(3):383–388. doi: 10.1016/0006-2952(93)90513-v. [DOI] [PubMed] [Google Scholar]
- DeBaun J. R., Miller E. C., Miller J. A. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970 Mar;30(3):577–595. [PubMed] [Google Scholar]
- Falany C. N., Vazquez M. E., Heroux J. A., Roth J. A. Purification and characterization of human liver phenol-sulfating phenol sulfotransferase. Arch Biochem Biophys. 1990 May 1;278(2):312–318. doi: 10.1016/0003-9861(90)90265-z. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Foldes A., Meek J. L. Occurrence and localization of brain phenolsulphotransferase. J Neurochem. 1974 Aug;23(2):303–307. doi: 10.1111/j.1471-4159.1974.tb04358.x. [DOI] [PubMed] [Google Scholar]
- Galinsky R. E., Manning B. W., Kimura R. E., Franklin M. R. Changes in conjugative enzyme activity and acetaminophen metabolism in young and senescent male F-344 rats following prolonged exposure to buthionine sulfoximine. Exp Gerontol. 1992;27(2):221–232. doi: 10.1016/0531-5565(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Gibby E. M., Cohen G. M. Conjugation of 1-naphthol by human bronchus and bronchoscopy samples. Biochem Pharmacol. 1984 Mar 1;33(5):739–743. doi: 10.1016/0006-2952(84)90456-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Orellana A., Gil G., Hirschberg C. B. Molecular cloning and expression of rat liver N-heparan sulfate sulfotransferase. J Biol Chem. 1992 Aug 5;267(22):15744–15750. [PubMed] [Google Scholar]
- Heroux J. A., Falany C. N., Roth J. A. Immunological characterization of human phenol sulfotransferase. Mol Pharmacol. 1989 Jul;36(1):29–33. [PubMed] [Google Scholar]
- Hirshey S. J., Dooley T. P., Reardon I. M., Heinrikson R. L., Falany C. N. Sequence analysis, in vitro translation, and expression of the cDNA for rat liver minoxidil sulfotransferase. Mol Pharmacol. 1992 Aug;42(2):257–264. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luks H. J., Spratt T. E., Vavrek M. T., Roland S. F., Weisburger J. H. Identification of sulfate and glucuronic acid conjugates of the 5-hydroxy derivative as major metabolites of 2-amino-3-methylimidazo[4,5-f]quinoline in rats. Cancer Res. 1989 Aug 15;49(16):4407–4411. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nash A. R., Glenn W. K., Moore S. S., Kerr J., Thompson A. R., Thompson E. O. Oestrogen sulfotransferase: molecular cloning and sequencing of cDNA for the bovine placental enzyme. Aust J Biol Sci. 1988;41(4):507–516. doi: 10.1071/bi9880507. [DOI] [PubMed] [Google Scholar]
- Otten M. H., Mol J. A., Visser T. J. Sulfation preceding deiodination of iodothyronines in rat hepatocytes. Science. 1983 Jul 1;221(4605):81–83. doi: 10.1126/science.6857270. [DOI] [PubMed] [Google Scholar]
- Otterness D. M., Wieben E. D., Wood T. C., Watson W. G., Madden B. J., McCormick D. J., Weinshilboum R. M. Human liver dehydroepiandrosterone sulfotransferase: molecular cloning and expression of cDNA. Mol Pharmacol. 1992 May;41(5):865–872. [PubMed] [Google Scholar]
- Ozawa S., Nagata K., Gong D. W., Yamazoe Y., Kato R. Expression and functional characterization of a rat sulfotransferase (ST1A1) cDNA for sulfations of phenolic substrates in COS-1 cells. Jpn J Pharmacol. 1993 Feb;61(2):153–156. doi: 10.1254/jjp.61.153. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Nagata K., Gong D. W., Yamazoe Y., Kato R. Nucleotide sequence of a full-length cDNA (PST-1) for aryl sulfotransferase from rat liver. Nucleic Acids Res. 1990 Jul 11;18(13):4001–4001. doi: 10.1093/nar/18.13.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer D. P., Norton T. R., Howell B. A. 2-Acetylaminofluorene-mediated alteration in the level of liver arylsulfotransferase IV during rat hepatocarcinogenesis. Cancer Res. 1990 Sep 1;50(17):5301–5307. [PubMed] [Google Scholar]
- Romberger D. J., Beckmann J. D., Claassen L., Ertl R. F., Rennard S. I. Modulation of fibronectin production of bovine bronchial epithelial cells by transforming growth factor-beta. Am J Respir Cell Mol Biol. 1992 Aug;7(2):149–155. doi: 10.1165/ajrcmb/7.2.149. [DOI] [PubMed] [Google Scholar]
- Roochvarg L. B., Thurman R. G., Kauffman F. C. 7-Ethoxycoumarin metabolism in hepatocytes from pre- and postpubescent male rats. Dev Pharmacol Ther. 1992;18(1-2):81–88. [PubMed] [Google Scholar]
- Runge-Morris M., Wilusz J. Age and gender-related gene expression of hydroxysteroid sulfotransferase-a in rat liver. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1051–1056. doi: 10.1016/0006-291x(91)91671-x. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Duffel M. W., Jakoby W. B. Aryl sulfotransferases. Methods Enzymol. 1981;77:197–206. doi: 10.1016/s0076-6879(81)77026-5. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Marcus C. J., Lyon E. S., Jakoby W. B. Assay of sulfotransferases. Anal Biochem. 1979 May;95(1):82–86. doi: 10.1016/0003-2697(79)90188-x. [DOI] [PubMed] [Google Scholar]
- Sundaram R. S., Szumlanski C., Otterness D., van Loon J. A., Weinshilboum R. M. Human intestinal phenol sulfotransferase: assay conditions, activity levels and partial purification of the thermolabile form. Drug Metab Dispos. 1989 May-Jun;17(3):255–264. [PubMed] [Google Scholar]
- Surh Y. J., Kwon H., Tannenbaum S. R. Sulfotransferase-mediated activation of 4-hydroxy- and 3,4-dihydroxy-3,4-dihydrocyclopenta[c,d]pyrene, major metabolites of cyclopenta[c,d]pyrene. Cancer Res. 1993 Mar 1;53(5):1017–1022. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. S., Nerland D. E. Hematotoxicity and concentration-dependent conjugation of phenol in mice following inhalation exposure to benzene. Toxicol Lett. 1991 Apr;56(1-2):159–166. doi: 10.1016/0378-4274(91)90102-c. [DOI] [PubMed] [Google Scholar]
- Whittemore R. M., Pearce L. B., Roth J. A. Purification and kinetic characterization of a dopamine-sulfating form of phenol sulfotransferase from human brain. Biochemistry. 1985 May 7;24(10):2477–2482. doi: 10.1021/bi00331a013. [DOI] [PubMed] [Google Scholar]
- Wilborn T. W., Comer K. A., Dooley T. P., Reardon I. M., Heinrikson R. L., Falany C. N. Sequence analysis and expression of the cDNA for the phenol-sulfating form of human liver phenol sulfotransferase. Mol Pharmacol. 1993 Jan;43(1):70–77. [PubMed] [Google Scholar]
- Wood T. C., Aksoy I. A., Aksoy S., Weinshilboum R. M. Human liver thermolabile phenol sulfotransferase: cDNA cloning, expression and characterization. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1119–1127. doi: 10.1006/bbrc.1994.1159. [DOI] [PubMed] [Google Scholar]
- Zhu X., Veronese M. E., Bernard C. C., Sansom L. N., McManus M. E. Identification of two human brain aryl sulfotransferase cDNAs. Biochem Biophys Res Commun. 1993 Aug 31;195(1):120–127. doi: 10.1006/bbrc.1993.2018. [DOI] [PubMed] [Google Scholar]