Abstract
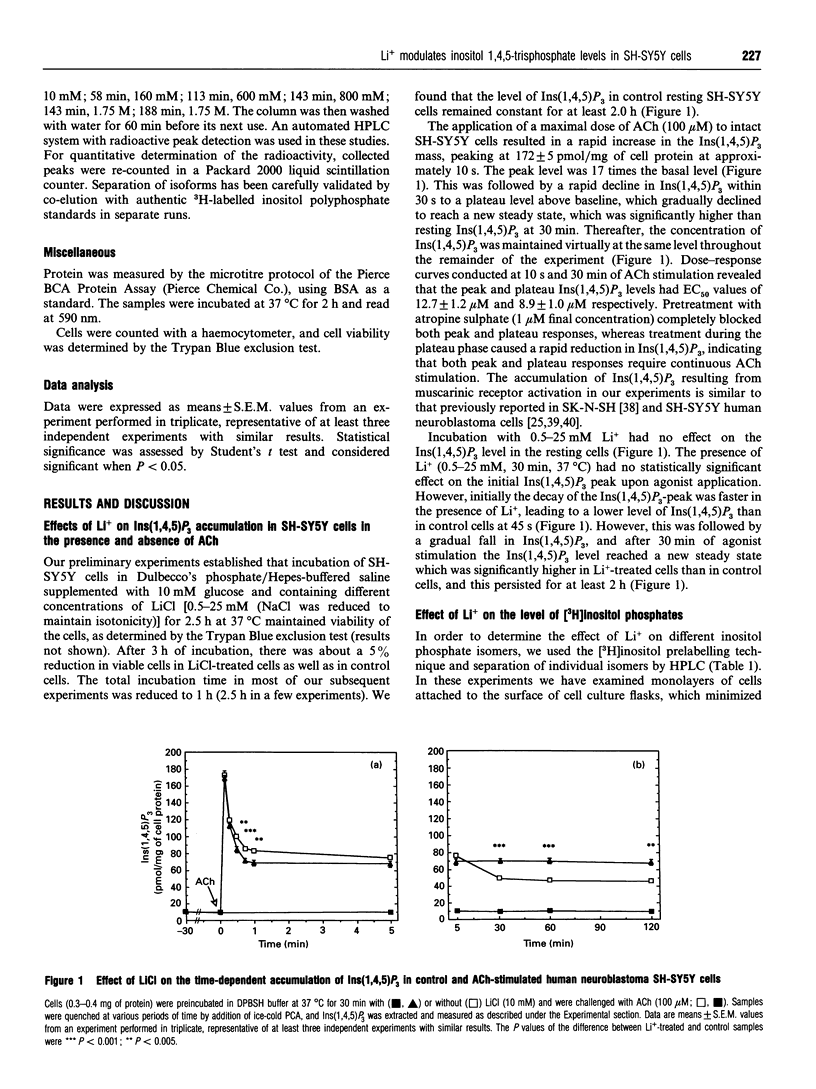
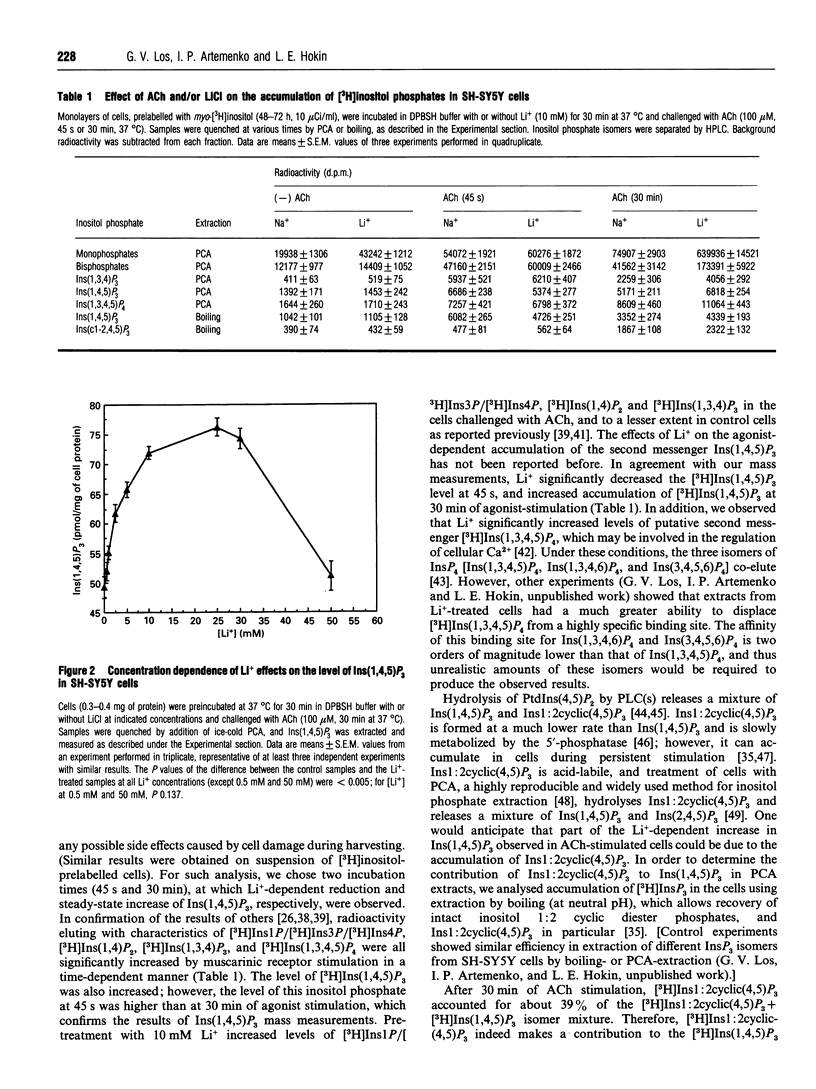
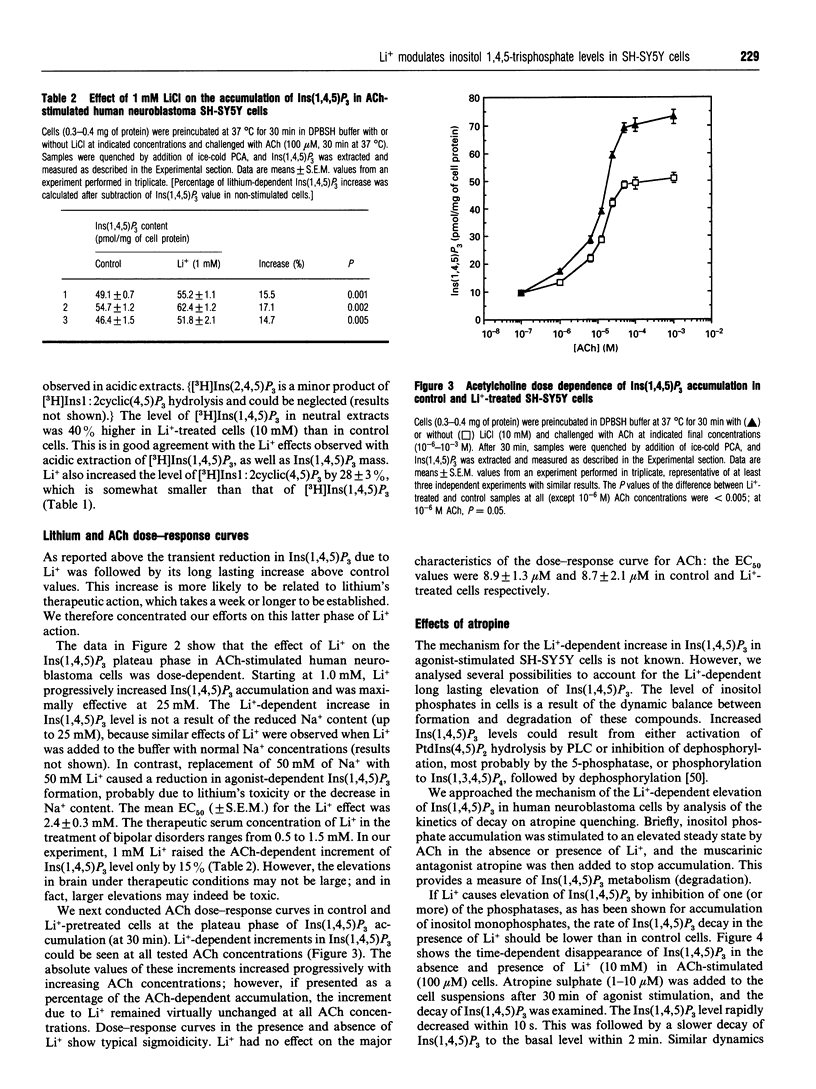
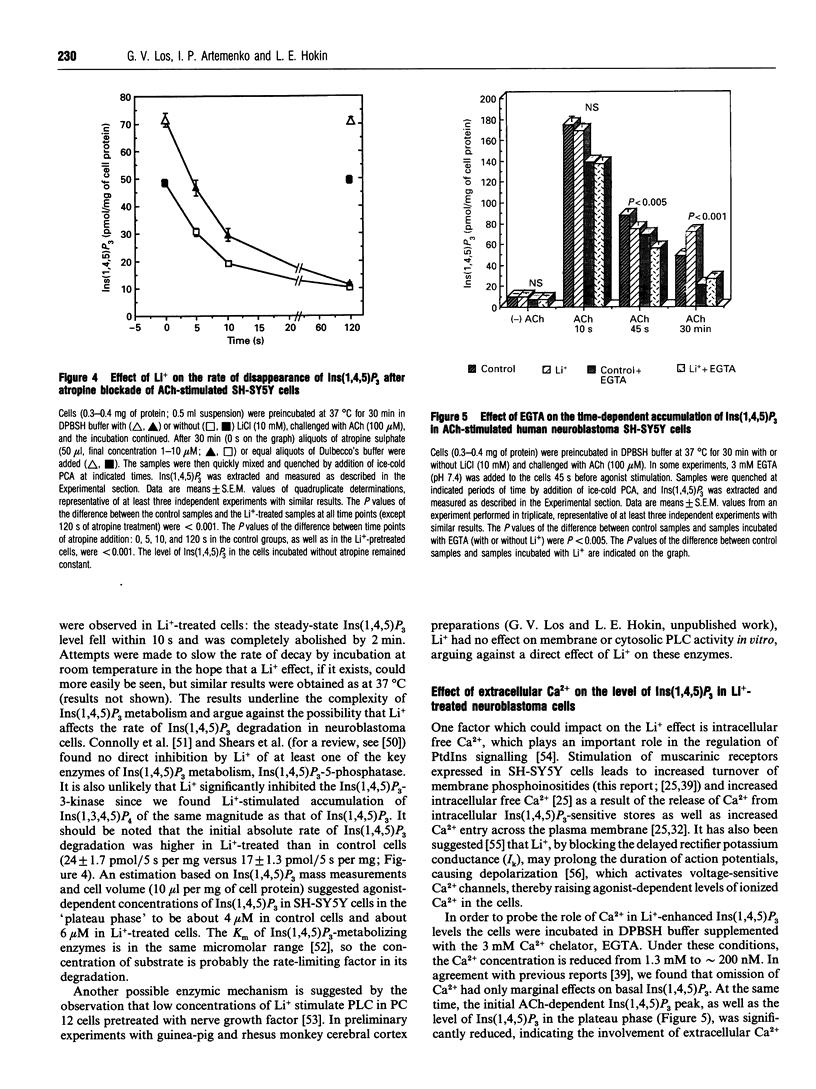
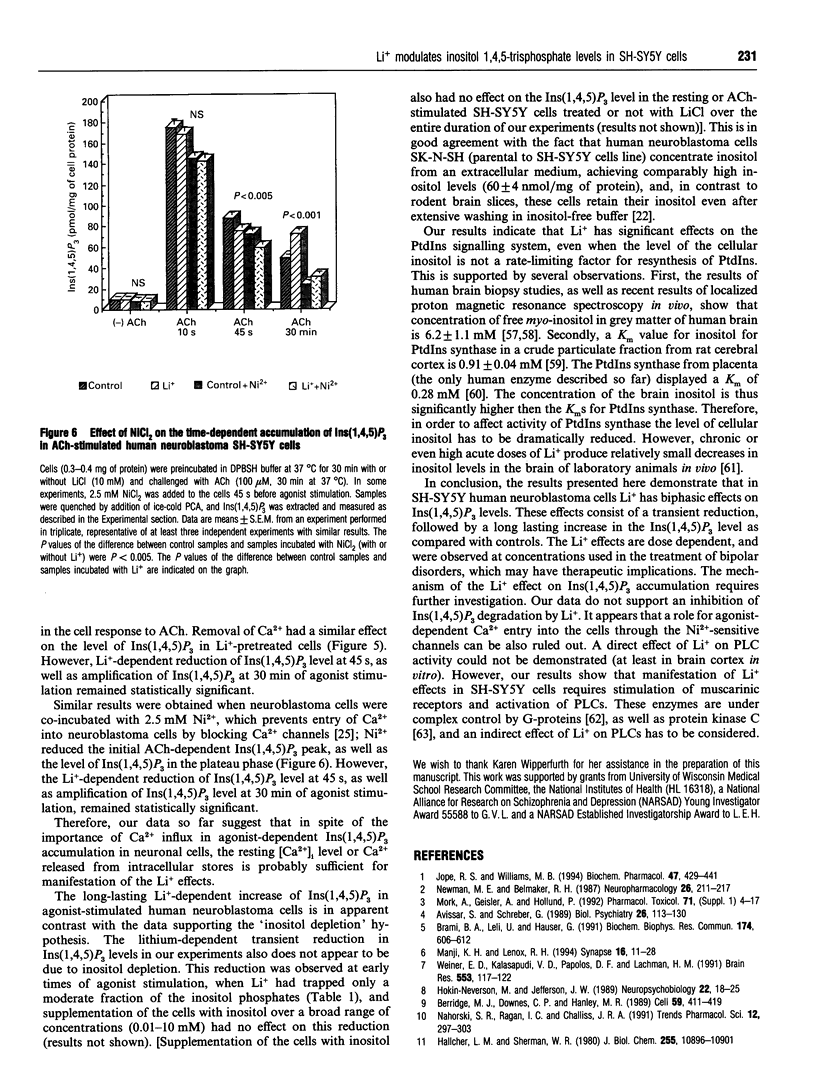
In order to approach the molecular mechanism of Li+'s mood-stabilizing action, the effect of Li+ (LiCl) on inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] mass was investigated in human neuroblastoma SH-SY5Y cells, which express muscarinic M3 receptors, coupled to PtdIns hydrolysis. Stimulation of these cells, with the cholinergic agonist acetylcholine, resulted in a rapid and transient increase in Ins(1,4,5)P3 with a maximum at 10 s. This was followed by a rapid decline in Ins(1,4,5)P3 within 30 s to a plateau level above baseline, which gradually declined to reach a new steady state, which was significantly higher than resting Ins(1,4,5)P3 at 30 min. Li+ had no effect on Ins(1,4,5)P3 in resting cells, as well as on the acetylcholine-dependent peak of Ins(1,4,5)P3. However, Li+ caused a transient reduction (at 45 s), followed by a long lasting increase in the Ins(1,4,5)P3 (30 min), as compared with controls. The Li+ effects were dose-dependent and were observed at concentrations used in the treatment of bipolar disorders. Supplementation with inositol had no effect on the level of Ins(1,4,5)P3, at least over the time periods studied. Stimulation of muscarinic receptors with consequent activation of phospholipase C were necessary for the manifestation of Li+ effects in SH-SY5Y cells, Li+ did not interfere with degradation of Ins(1,4,5)P3 after receptor-blockade with atropine, suggesting that Li+ has no direct effect on the Ins(1,4,5)P3-metabolizing enzymes. A direct effect of Li+ on the phospholipase C also is unlikely. Blockade of Ca2+ entry into the cells by Ni2+, or incubation with EGTA, which reduces agonist-stimulated accumulation of Ins(1,4,5)P3, had no effect on the Li(+)-dependent increase in Ins(1,4,5)P3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam-Vizi V., Banay-Schwartz M., Wajda I., Lajtha A. Depolarization of brain cortex slices and synaptosomes by lithium. Determination of K+-equilibrium potential in cortex slices. Brain Res. 1987 May 5;410(2):257–263. doi: 10.1016/0006-8993(87)90322-2. [DOI] [PubMed] [Google Scholar]
- Akerman K. E. Depolarization of human neuroblastoma cells as a result of muscarinic receptor-induced rise in cytosolic Ca2+. FEBS Lett. 1989 Jan 2;242(2):337–340. doi: 10.1016/0014-5793(89)80497-1. [DOI] [PubMed] [Google Scholar]
- Ammer H., Schulz R. Alterations in the expression of G-proteins and regulation of adenylate cyclase in human neuroblastoma SH-SY5Y cells chronically exposed to low-efficacy mu-opioids. Biochem J. 1993 Oct 1;295(Pt 1):263–271. doi: 10.1042/bj2950263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B. E. Purification and characterization of phosphatidylinositol synthase from human placenta. Biochem J. 1994 Feb 1;297(Pt 3):517–522. doi: 10.1042/bj2970517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack J. R., Prior A. M., Griffith D., Ragan C. I. Characterization of the effects of lithium on phosphatidylinositol (PI) cycle activity in human muscarinic m1 receptor-transfected CHO cells. Br J Pharmacol. 1993 Oct;110(2):809–815. doi: 10.1111/j.1476-5381.1993.tb13884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar S., Schreiber G. Muscarinic receptor subclassification and G-proteins: significance for lithium action in affective disorders and for the treatment of the extrapyramidal side effects of neuroleptics. Biol Psychiatry. 1989 Jun;26(2):113–130. doi: 10.1016/0006-3223(89)90015-2. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Brami B. A., Leli U., Hauser G. Influence of lithium on second messenger accumulation in NG108-15 cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):606–612. doi: 10.1016/0006-291x(91)91460-t. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Connolly T. M., Wilson D. B., Bross T. E., Majerus P. W. Isolation and characterization of the inositol cyclic phosphate products of phosphoinositide cleavage by phospholipase C. Metabolism in cell-free extracts. J Biol Chem. 1986 Jan 5;261(1):122–126. [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaux A., Erneux C., Moreau C., Dumont J. E. Enzymic dephosphorylation of D-myo-inositol 1,4-bisphosphate in rat brain. Biochem J. 1987 Feb 15;242(1):193–198. doi: 10.1042/bj2420193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Inositol 1,2-cyclic 4,5-trisphosphate concentration relative to inositol 1,4,5-trisphosphate in pancreatic minilobules on stimulation with carbamylcholine in the absence of lithium. Possible role as a second messenger in long- but not short-term responses. J Biol Chem. 1987 Oct 15;262(29):13892–13895. [PubMed] [Google Scholar]
- Dixon J. F., Lee C. H., Los G. V., Hokin L. E. Lithium enhances accumulation of [3H]inositol radioactivity and mass of second messenger inositol 1,4,5-trisphosphate in monkey cerebral cortex slices. J Neurochem. 1992 Dec;59(6):2332–2335. doi: 10.1111/j.1471-4159.1992.tb10129.x. [DOI] [PubMed] [Google Scholar]
- Dixon J. F., Los G. V., Hokin L. E. Lithium stimulates glutamate "release" and inositol 1,4,5-trisphosphate accumulation via activation of the N-methyl-D-aspartate receptor in monkey and mouse cerebral cortex slices. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8358–8362. doi: 10.1073/pnas.91.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Ragan C. I., Watling K. J., Aspley S., Jackson R. G., Reid G. G., Gani D., Shute J. K. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochem J. 1988 Feb 1;249(3):883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Reid G. G., Jackson R. G., Barnaby R. J., Ragan C. I. Purification and properties of inositol-1,4-bisphosphatase from bovine brain. Biochem J. 1988 Aug 1;253(3):777–782. doi: 10.1042/bj2530777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hawkins P. T., Berrie C. P., Morris A. J., Downes C. P. Inositol 1,2-cyclic 4,5-trisphosphate is not a product of muscarinic receptor-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis in rat parotid glands. Biochem J. 1987 Apr 1;243(1):211–218. doi: 10.1042/bj2430211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock A. M., Seguin E. B., Agranoff B. W. Measurement of receptor-activated phosphoinositide turnover in rat brain: nonequivalence of inositol phosphate and CDP-diacylglycerol formation. J Neurochem. 1993 Mar;60(3):1087–1092. doi: 10.1111/j.1471-4159.1993.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Hokin-Neaverson M., Jefferson J. W. Deficient erythrocyte NaK-ATPase activity in different affective states in bipolar affective disorder and normalization by lithium therapy. Neuropsychobiology. 1989;22(1):18–25. doi: 10.1159/000118587. [DOI] [PubMed] [Google Scholar]
- Hughes A. R., Takemura H., Putney J. W., Jr Kinetics of inositol 1,4,5-trisphosphate and inositol cyclic 1:2,4,5-trisphosphate metabolism in intact rat parotid acinar cells. Relationship to calcium signalling. J Biol Chem. 1988 Jul 25;263(21):10314–10319. [PubMed] [Google Scholar]
- Hughes P. J., Michell R. H. Novel inositol containing phospholipids and phosphates: their synthesis and possible new roles in cellular signalling. Curr Opin Neurobiol. 1993 Jun;3(3):383–400. doi: 10.1016/0959-4388(93)90132-i. [DOI] [PubMed] [Google Scholar]
- Jenkinson S., Patel N., Nahorski S. R., Challiss R. A. Comparative effects of lithium on the phosphoinositide cycle in rat cerebral cortex, hippocampus, and striatum. J Neurochem. 1993 Sep;61(3):1082–1090. doi: 10.1111/j.1471-4159.1993.tb03623.x. [DOI] [PubMed] [Google Scholar]
- Jope R. S., Williams M. B. Lithium and brain signal transduction systems. Biochem Pharmacol. 1994 Feb 9;47(3):429–441. doi: 10.1016/0006-2952(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Kanba S., Yagi G., Nakaki T., Kato R., Richelson E. Potentiation by a sodium channel activator of effects of lithium ion on cyclic AMP, cyclic GMP and inositol phosphates. Neuropharmacology. 1991 May;30(5):497–500. doi: 10.1016/0028-3908(91)90012-z. [DOI] [PubMed] [Google Scholar]
- Kato M., Lledo P. M., Vincent J. D. Blockade by lithium ions of potassium channels in rat anterior pituitary cells. Am J Physiol. 1991 Aug;261(2 Pt 1):C218–C223. doi: 10.1152/ajpcell.1991.261.2.C218. [DOI] [PubMed] [Google Scholar]
- Kazmi S. M., Mishra R. K. Opioid receptors in human neuroblastoma SH-SY5Y cells: evidence for distinct morphine (mu) and enkephalin (delta) binding sites. Biochem Biophys Res Commun. 1986 Jun 13;137(2):813–820. doi: 10.1016/0006-291x(86)91152-6. [DOI] [PubMed] [Google Scholar]
- Kennedy E. D., Challiss R. A., Ragan C. I., Nahorski S. R. Reduced inositol polyphosphate accumulation and inositol supply induced by lithium in stimulated cerebral cortex slices. Biochem J. 1990 May 1;267(3):781–786. doi: 10.1042/bj2670781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Ryu S. H., Rhee S. G. Cyclic and noncyclic inositol phosphates are formed at different ratios by phospholipase C isozymes. Biochem Biophys Res Commun. 1989 Aug 30;163(1):177–182. doi: 10.1016/0006-291x(89)92117-7. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Challiss R. A., Nahorski S. R. Accumulation and metabolism of Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in muscarinic-receptor-stimulated SH-SY5Y neuroblastoma cells. Biochem J. 1991 Feb 1;273(Pt 3):791–794. doi: 10.1042/bj2730791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. G., Nahorski S. R. Muscarinic-receptor-mediated changes in intracellular Ca2+ and inositol 1,4,5-trisphosphate mass in a human neuroblastoma cell line, SH-SY5Y. Biochem J. 1990 Jan 15;265(2):555–562. doi: 10.1042/bj2650555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. G., Whitham E. M., Baird J. G., Nahorski S. R. Different mechanisms of Ca2+ entry induced by depolarization and muscarinic receptor stimulation in SH-SY5Y human neuroblastoma cells. Brain Res Mol Brain Res. 1990 Aug;8(3):263–266. doi: 10.1016/0169-328x(90)90026-a. [DOI] [PubMed] [Google Scholar]
- Larsson C., Simonsson P. Desensitization of acetylcholine induced inositol 1,4,5-trisphosphate formation in neuroblastoma SH-SY5Y cells following repetitive acetylcholine stimulations. Neurosci Lett. 1993 Feb 19;150(2):141–144. doi: 10.1016/0304-3940(93)90521-l. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Dixon J. F., Reichman M., Moummi C., Los G., Hokin L. E. Li+ increases accumulation of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate in cholinergically stimulated brain cortex slices in guinea pig, mouse and rat. The increases require inositol supplementation in mouse and rat but not in guinea pig. Biochem J. 1992 Mar 1;282(Pt 2):377–385. doi: 10.1042/bj2820377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leli U., Shea T. B., Cataldo A., Hauser G., Grynspan F., Beermann M. L., Liepkalns V. A., Nixon R. A., Parker P. J. Differential expression and subcellular localization of protein kinase C alpha, beta, gamma, delta, and epsilon isoforms in SH-SY5Y neuroblastoma cells: modifications during differentiation. J Neurochem. 1993 Jan;60(1):289–298. doi: 10.1111/j.1471-4159.1993.tb05850.x. [DOI] [PubMed] [Google Scholar]
- Manji H. K., Lenox R. H. Long-term action of lithium: a role for transcriptional and posttranscriptional factors regulated by protein kinase C. Synapse. 1994 Jan;16(1):11–28. doi: 10.1002/syn.890160103. [DOI] [PubMed] [Google Scholar]
- Martin D. M., Yee D., Carlson R. O., Feldman E. L. Gene expression of the insulin-like growth factors and their receptors in human neuroblastoma cell lines. Brain Res Mol Brain Res. 1992 Oct;15(3-4):241–246. doi: 10.1016/0169-328x(92)90114-q. [DOI] [PubMed] [Google Scholar]
- Michaelis T., Merboldt K. D., Bruhn H., Hänicke W., Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology. 1993 Apr;187(1):219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- Mørk A., Geisler A., Hollund P. Effects of lithium on second messenger systems in the brain. Pharmacol Toxicol. 1992;71 (Suppl 1):4–17. doi: 10.1111/j.1600-0773.1992.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Ragan C. I., Challiss R. A. Lithium and the phosphoinositide cycle: an example of uncompetitive inhibition and its pharmacological consequences. Trends Pharmacol Sci. 1991 Aug;12(8):297–303. doi: 10.1016/0165-6147(91)90581-c. [DOI] [PubMed] [Google Scholar]
- Newman M. E., Belmaker R. H. Effects of lithium in vitro and ex vivo on components of the adenylate cyclase system in membranes from the cerebral cortex of the rat. Neuropharmacology. 1987 Feb-Mar;26(2-3):211–217. doi: 10.1016/0028-3908(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Petroff O. A., Spencer D. D., Alger J. R., Prichard J. W. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989 Sep;39(9):1197–1202. doi: 10.1212/wnl.39.9.1197. [DOI] [PubMed] [Google Scholar]
- Reeve H. L., Vaughan P. F., Peers C. Glibenclamide inhibits a voltage-gated K+ current in the human neuroblastoma cell line SH-SY5Y. Neurosci Lett. 1992 Jan 20;135(1):37–40. doi: 10.1016/0304-3940(92)90130-y. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Multiple forms of phospholipase C isozymes and their activation mechanisms. Adv Second Messenger Phosphoprotein Res. 1992;26:35–61. [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Ruiz-Larrea F., Drummond A. H. Pathways of dephosphorylation of 1-D-myo-inositol 1,4,5-trisphosphate in GH3 pituitary tumor cells. Biochim Biophys Acta. 1993 Jul 28;1178(1):63–72. doi: 10.1016/0167-4889(93)90110-b. [DOI] [PubMed] [Google Scholar]
- Serra M., Mei L., Roeske W. R., Lui G. K., Watson M., Yamamura H. I. The intact human neuroblastoma cell (SH-SY5Y) exhibits high-affinity [3H]pirenzepine binding associated with hydrolysis of phosphatidylinositols. J Neurochem. 1988 May;50(5):1513–1521. doi: 10.1111/j.1471-4159.1988.tb03038.x. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Metabolism of inositol phosphates. Adv Second Messenger Phosphoprotein Res. 1992;26:63–92. [PubMed] [Google Scholar]
- Sherman W. R., Gish B. G., Honchar M. P., Munsell L. Y. Effects of lithium on phosphoinositide metabolism in vivo. Fed Proc. 1986 Oct;45(11):2639–2646. [PubMed] [Google Scholar]
- Stubbs E. B., Jr, Agranoff B. W. Lithium enhances muscarinic receptor-stimulated CDP-diacylglycerol formation in inositol-depleted SK-N-SH neuroblastoma cells. J Neurochem. 1993 Apr;60(4):1292–1299. doi: 10.1111/j.1471-4159.1993.tb03289.x. [DOI] [PubMed] [Google Scholar]
- Volonté C., Racker E. Lithium stimulation of membrane-bound phospholipase C from PC12 cells exposed to nerve growth factor. J Neurochem. 1988 Oct;51(4):1163–1168. doi: 10.1111/j.1471-4159.1988.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Weiner E. D., Kalasapudi V. D., Papolos D. F., Lachman H. M. Lithium augments pilocarpine-induced fos gene expression in rat brain. Brain Res. 1991 Jul 5;553(1):117–122. doi: 10.1016/0006-8993(91)90238-q. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Sherman W. R., Berger R. A., Majerus P. W. Inositol cyclic phosphates are produced by cleavage of phosphatidylphosphoinositols (polyphosphoinositides) with purified sheep seminal vesicle phospholipase C enzymes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4013–4017. doi: 10.1073/pnas.82.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]