Abstract
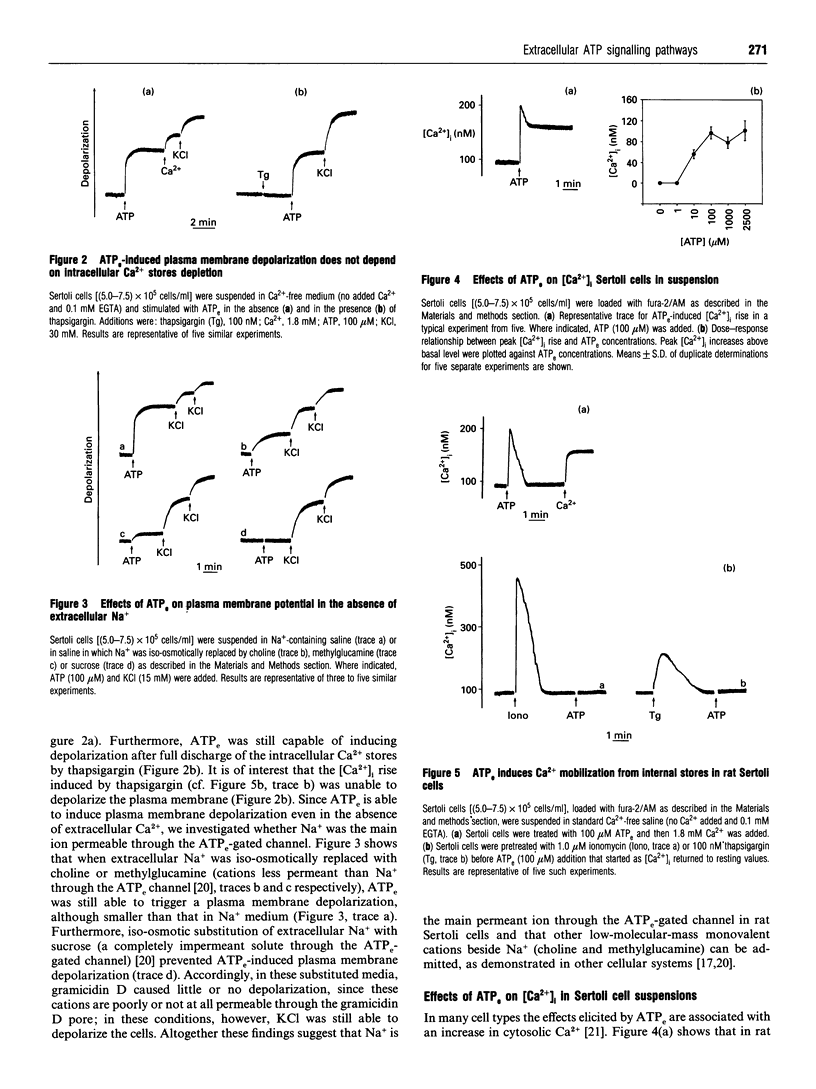
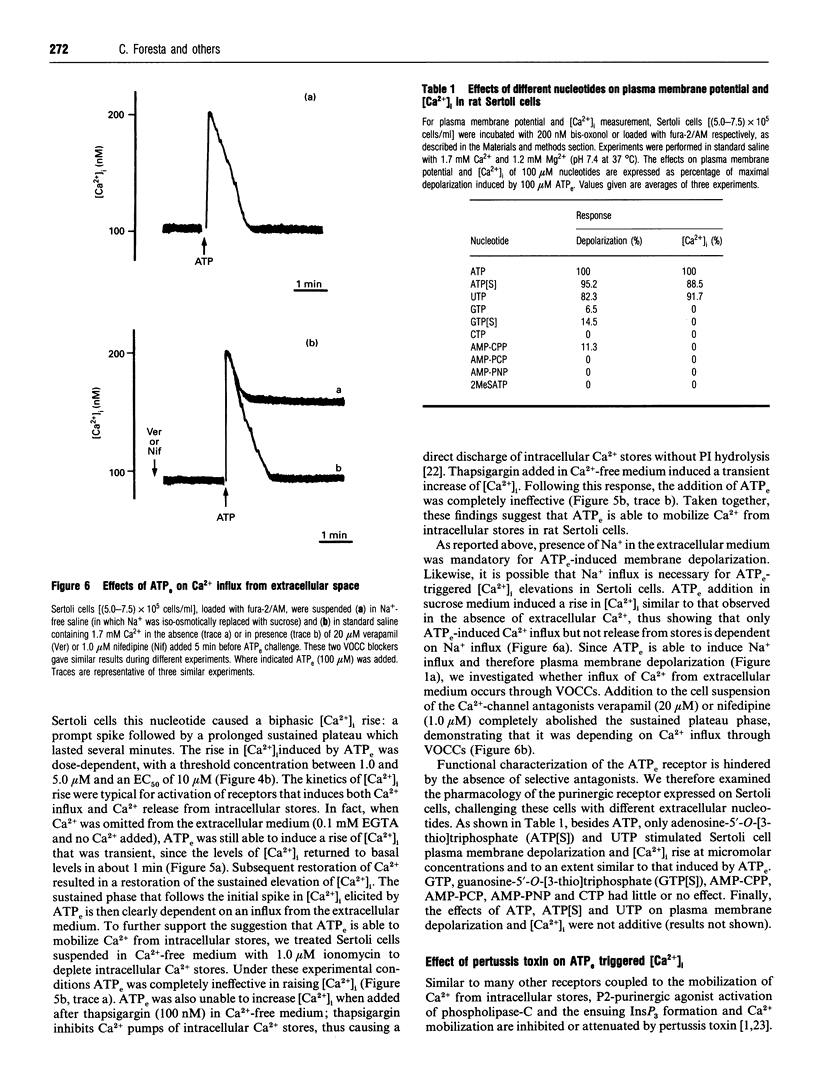
1. The present study describes effects of extracellular ATP (ATPe) on plasma membrane potential and cytoplasmic Ca2+ concentrations ([Ca2+]i) in rat Sertoli cells. Sertoli cells in suspension were stimulated with ATPe and other nucleotides and ionic changes were monitored utilizing the fluorescent dyes bis-oxonol and fura-2/AM. ATPe induced a prompt plasma membrane depolarization which was dependent on Na+ influx from the extracellular medium, since it was abolished by omission of extracellular Na+. Depolarization was independent of [Ca2+]i rise as it also occurred in the absence of extracellular Ca2+ and after intracellular Ca2+ stores were discharged with thapsigargin. ATPe also stimulated a rapid and biphasic increase in [Ca2+]i: a prompt spike was followed by a prolonged sustained plateau. The initial spike was dependent on Ca2+ release from intracellular stores since it was also present when cells were incubated in EGTA-supplemented Ca(2+)-free medium and was abolished by pretreatment with ionomycin and thapsigargin, agents that discharge intracellular Ca2+ stores. The sustained phase was dependent on Ca2+ influx from the extracellular medium as it was abolished when cells were incubated in EGTA-supplemented Ca(2+)-free medium. Ca2+ influx was due to activation of voltage-operated calcium channels (VOCCs) since it was abolished by the VOCC inhibitors verapamil and nifedipine or incubation in sucrose medium, an experimental condition which precludes plasma membrane depolarization by ATPe. 2. ATPe-induced rises in intracellular Ca2+ concentration and plasma membrane depolarization were reduced by pretreatment with pertussis toxin, suggesting that ATPe-activated transduction mechanisms are in part under the control of pertussis toxin-sensitive G-proteins. These data show that Sertoli cells possess P2-purinergic receptor subtypes coupled to influx of Na+ and release of Ca2+ from intracellular stores and provide evidence for an activation of different pathways by extracellular ATPe. Activation of these receptors induces Na+ influx that causes a rapid plasma membrane depolarization. Furthermore, ATPe also triggers Ca2+ release from intracellular stores and Ca2+ influx from extracellular space via dihydropyridine-sensitive VOCCs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Christie A., Sharma V. K., Sheu S. S. Mechanism of extracellular ATP-induced increase of cytosolic Ca2+ concentration in isolated rat ventricular myocytes. J Physiol. 1992 Jan;445:369–388. doi: 10.1113/jphysiol.1992.sp018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen D. S., Baker B., Dubyak G. R. Pertussis toxin produces differential inhibitory effects on basal, P2-purinergic, and chemotactic peptide-stimulated inositol phospholipid breakdown in HL-60 cells and HL-60 cell membranes. J Biol Chem. 1990 Sep 25;265(27):16181–16189. [PubMed] [Google Scholar]
- Di Virgilio F., Bronte V., Collavo D., Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5'-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol. 1989 Sep 15;143(6):1955–1960. [PubMed] [Google Scholar]
- Di Virgilio F., Meyer B. C., Greenberg S., Silverstein S. C. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J Cell Biol. 1988 Mar;106(3):657–666. doi: 10.1083/jcb.106.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R. Signal transduction by P2-purinergic receptors for extracellular ATP. Am J Respir Cell Mol Biol. 1991 Apr;4(4):295–300. doi: 10.1165/ajrcmb/4.4.295. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Filippini A., Riccioli A., De Cesaris P., Paniccia R., Teti A., Stefanini M., Conti M., Ziparo E. Activation of inositol phospholipid turnover and calcium signaling in rat Sertoli cells by P2-purinergic receptors: modulation of follicle-stimulating hormone responses. Endocrinology. 1994 Mar;134(3):1537–1545. doi: 10.1210/endo.134.3.8119196. [DOI] [PubMed] [Google Scholar]
- Foresta C., Rossato M., Di Virgilio F. Extracellular ATP is a trigger for the acrosome reaction in human spermatozoa. J Biol Chem. 1992 Sep 25;267(27):19443–19447. [PubMed] [Google Scholar]
- Foresta C., Rossato M., Di Virgilio F. Ion fluxes through the progesterone-activated channel of the sperm plasma membrane. Biochem J. 1993 Aug 15;294(Pt 1):279–283. doi: 10.1042/bj2940279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Garritsen A., Zhang Y., Cooper D. M. Purinergic receptor regulation of signal transduction in NCB-20 cells. Mol Pharmacol. 1992 Apr;41(4):743–749. [PubMed] [Google Scholar]
- Gorczynska E., Handelsman D. J. The role of calcium in follicle-stimulating hormone signal transduction in Sertoli cells. J Biol Chem. 1991 Dec 15;266(35):23739–23744. [PubMed] [Google Scholar]
- Harden T. K., Boyer J. L., Brown H. A., Cooper C. L., Jeffs R. A., Martin M. W. Biochemical properties of a P2Y-purinergic receptor. Ann N Y Acad Sci. 1990;603:256–266. doi: 10.1111/j.1749-6632.1990.tb37677.x. [DOI] [PubMed] [Google Scholar]
- Li G. D., Milani D., Dunne M. J., Pralong W. F., Theler J. M., Petersen O. H., Wollheim C. B. Extracellular ATP causes Ca2(+)-dependent and -independent insulin secretion in RINm5F cells. Phospholipase C mediates Ca2+ mobilization but not Ca2+ influx and membrane depolarization. J Biol Chem. 1991 Feb 25;266(6):3449–3457. [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco L., DeManno D. A., Martin M. W., Conti M. Adenosine inhibition of the hormonal response in the Sertoli cell is reversed by pertussis toxin. Endocrinology. 1988 Jun;122(6):2692–2698. doi: 10.1210/endo-122-6-2692. [DOI] [PubMed] [Google Scholar]
- Monaco L., Toscano M. V., Conti M. Purine modulation of the hormonal response of the rat Sertoli cell in culture. Endocrinology. 1984 Oct;115(4):1616–1624. doi: 10.1210/endo-115-4-1616. [DOI] [PubMed] [Google Scholar]
- Morley P., Vanderhyden B. C., Tremblay R., Mealing G. A., Durkin J. P., Whitfield J. F. Purinergic receptor-mediated intracellular Ca2+ oscillations in chicken granulosa cells. Endocrinology. 1994 Mar;134(3):1269–1276. doi: 10.1210/endo.134.3.8119167. [DOI] [PubMed] [Google Scholar]
- Murgia M., Hanau S., Pizzo P., Rippa M., Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993 Apr 15;268(11):8199–8203. [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Pizzo P., Zanovello P., Bronte V., Di Virgilio F. Extracellular ATP causes lysis of mouse thymocytes and activates a plasma membrane ion channel. Biochem J. 1991 Feb 15;274(Pt 1):139–144. doi: 10.1042/bj2740139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell D. The effects of ATP and related nucleotides on visceral smooth muscle. Ann N Y Acad Sci. 1990;603:53–63. doi: 10.1111/j.1749-6632.1990.tb37661.x. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Talamo B. R. ATP activates a cation-permeable pathway in rat parotid acinar cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C934–C940. doi: 10.1152/ajpcell.1992.262.4.C934. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987 Jun 25;262(18):8884–8888. [PubMed] [Google Scholar]
- Steinberg T. H., Silverstein S. C. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987 Mar 5;262(7):3118–3122. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Moatassim C., Dornand J., Mani J. C. Extracellular ATP and cell signalling. Biochim Biophys Acta. 1992 Feb 19;1134(1):31–45. doi: 10.1016/0167-4889(92)90025-7. [DOI] [PubMed] [Google Scholar]


