Abstract
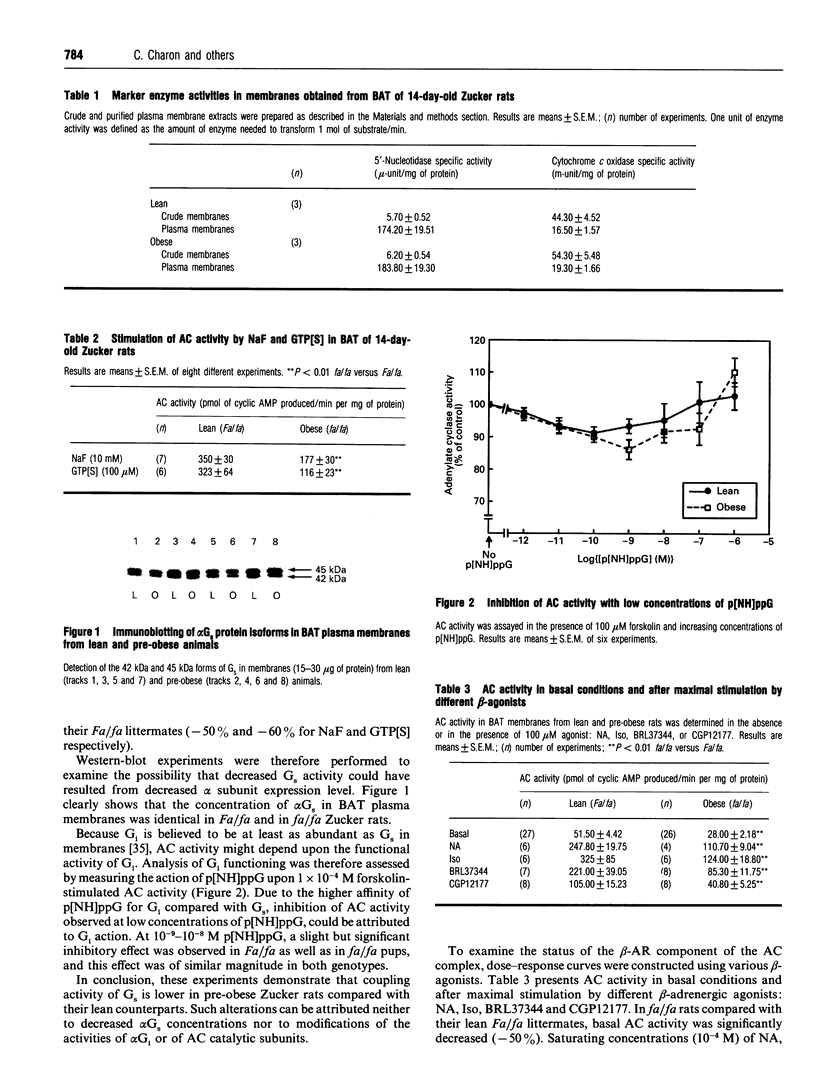
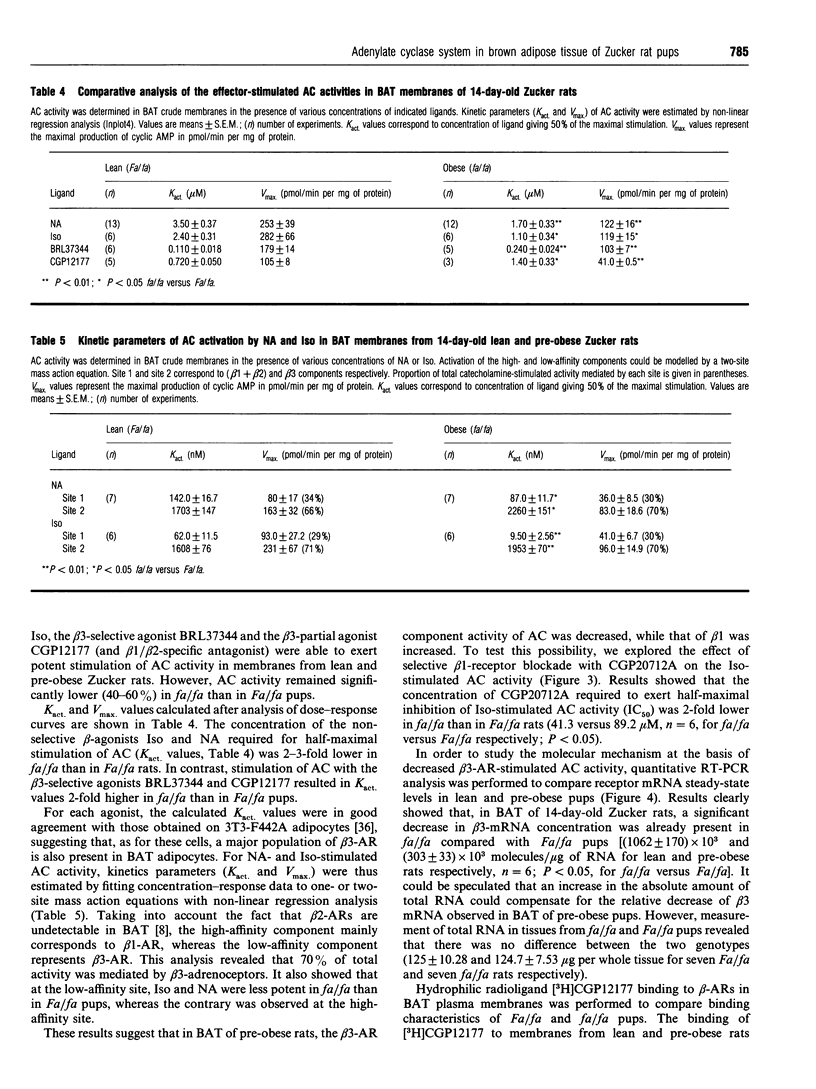
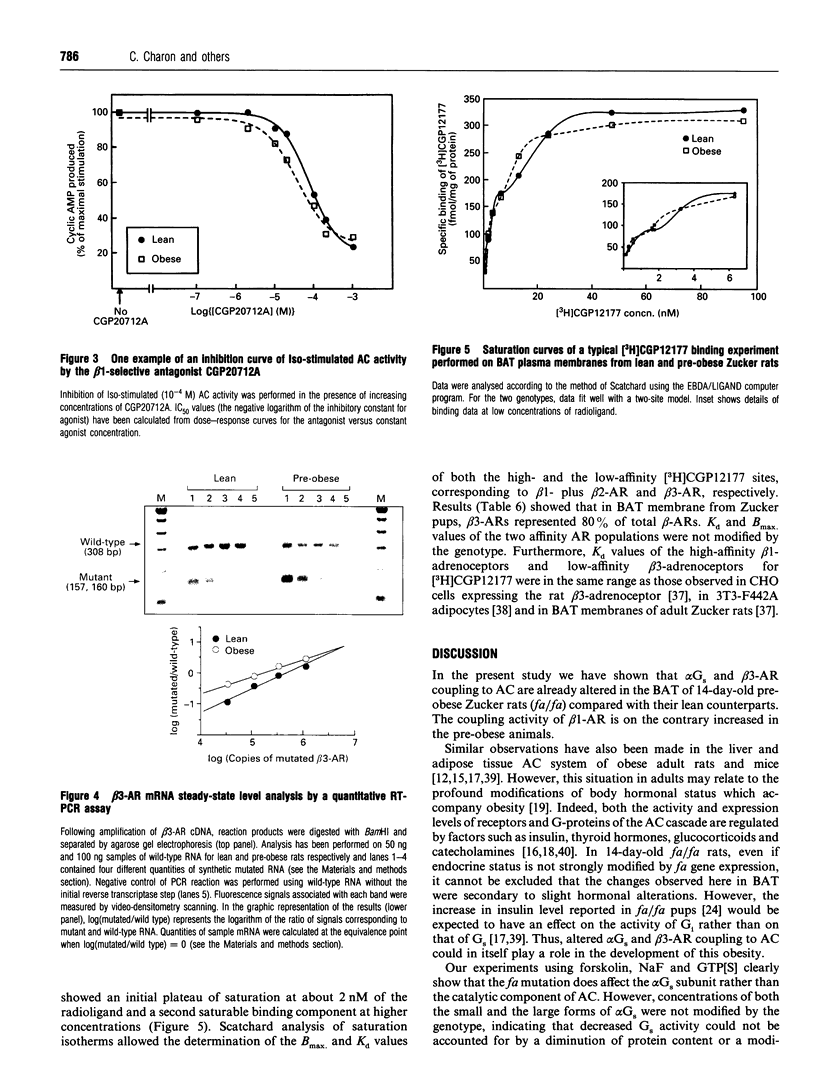
This study was undertaken to determine whether receptor and non-receptor components of the adenylate cyclase (AC) cascade were altered in brown adipose tissue (BAT) of 14-day-old pre-obese (fa/fa) rats, before endocrine status is strongly modified by fa gene expression. Activity of the AC catalytic subunit did not differ between the two genotypes. In fa/fa rats compared with control Fa/fa rats, there was a 50% decrease in the activity of alpha Gs (stimulated by NaF or guanosine 5'-[gamma-thio]triphosphate) but no change in protein content (Western blotting). alpha Gi function, assessed by the inhibitory action of low concentrations of guanosine 5'-[beta gamma-imido]triphosphate upon 10(-4) M forskolin-stimulated AC activity, was equally low in both genotypes. Analysis of dose-response curves for different beta-agonists revealed that (i) both the basal and the maximally stimulated activity of AC were 2-fold lower in fa/fa rats than in Fa/fa rats; (ii) BRL37344 and CGP12177 (beta 3 agonists) were less potent in fa/fa than in Fa/fa rats (Kact. multiplied by 2); (iii) noradrenaline and isoprenaline (Iso), at the low-affinity site (beta 3-AR), were less potent in fa/fa than in Fa/fa pups (Kact. increased by 30 and 20% respectively). At the high-affinity site (mainly beta 1) these two agonists were more potent in fa/fa than in Fa/fa rats (Kact. decreased by 40 and 80% respectively). In good agreement with the latter result, the beta 1-adrenergic receptor (beta 1-AR)-selective antagonist CGP20712A had more effect on the Iso-stimulated AC activity in pre-obese than in lean pups (2-fold decreased in IC50). Binding experiments with [3H]CGP12177 show that in BAT of suckling rats, beta 3-ARs represent 80% of the total beta-ARs. Bmax values for the two sites were not affected by the genotype, although the beta 3-AR mRNA concentration in BAT (quantitative reverse-transcriptase PCR) was 3-fold lower in fa/fa rats than in Fa/fa pups. In conclusion, these results provide evidence for alterations in beta 1- and beta 3-AR signalling in BAT of 14-day-old suckling pre-obese Zucker rats with a decreased activity of alpha Gs. The impaired AC responsiveness to catecholamines might be a primary contributor to the development of this genetic obesity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bazin R., Eteve D., Lavau M. Evidence for decreased GDP binding to brown-adipose-tissue mitochondria of obese Zucker (fa/fa) rats in the very first days of life. Biochem J. 1984 Jul 1;221(1):241–245. doi: 10.1042/bj2210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray G. A., York D. A. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979 Jul;59(3):719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- Bégin-Heick N. Absence of the inhibitory effect of guanine nucleotides on adenylate cyclase activity in white adipocyte membranes of the ob/ob mouse. Effect of the ob gene. J Biol Chem. 1985 May 25;260(10):6187–6193. [PubMed] [Google Scholar]
- Bégin-Heick N. Alpha-subunits of Gs and Gi in adipocyte plasma membranes of genetically diabetic (db/db) mice. Am J Physiol. 1992 Jul;263(1 Pt 1):C121–C129. doi: 10.1152/ajpcell.1992.263.1.C121. [DOI] [PubMed] [Google Scholar]
- Bégin-Heick N. Quantification of the alpha and beta subunits of the transducing elements (Gs and Gi) of adenylate cyclase in adipocyte membranes from lean and obese (ob/ob) mice. Biochem J. 1990 May 15;268(1):83–89. doi: 10.1042/bj2680083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A., Granneman J. G. Developmental changes in adenylyl cyclase and GTP binding proteins in brown fat. Am J Physiol. 1991 Aug;261(2 Pt 2):R403–R411. doi: 10.1152/ajpregu.1991.261.2.R403. [DOI] [PubMed] [Google Scholar]
- Chaudhry A., MacKenzie R. G., Georgic L. M., Granneman J. G. Differential interaction of beta 1- and beta 3-adrenergic receptors with Gi in rat adipocytes. Cell Signal. 1994 May;6(4):457–465. doi: 10.1016/0898-6568(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Collins S., Daniel K. W., Rohlfs E. M., Ramkumar V., Taylor I. L., Gettys T. W. Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol Endocrinol. 1994 Apr;8(4):518–527. doi: 10.1210/mend.8.4.7914350. [DOI] [PubMed] [Google Scholar]
- D'Allaire F., Atgié C., Mauriège P., Simard P. M., Bukowiecki L. J. Characterization of beta 1- and beta 3-adrenoceptors in intact brown adipocytes of the rat. Br J Pharmacol. 1995 Jan;114(2):275–282. doi: 10.1111/j.1476-5381.1995.tb13223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Engfeldt P., Hellmér J., Wahrenberg H., Arner P. Effects of insulin on adrenoceptor binding and the rate of catecholamine-induced lipolysis in isolated human fat cells. J Biol Chem. 1988 Oct 25;263(30):15553–15560. [PubMed] [Google Scholar]
- Fève B., Elhadri K., Quignard-Boulangé A., Pairault J. Transcriptional down-regulation by insulin of the beta 3-adrenergic receptor expression in 3T3-F442A adipocytes: a mechanism for repressing the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5677–5681. doi: 10.1073/pnas.91.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Briend-Sutren M. M., Lasnier F., Strosberg A. D., Pairault J. Differential regulation of beta 1- and beta 2-adrenergic receptor protein and mRNA levels by glucocorticoids during 3T3-F442A adipose differentiation. J Biol Chem. 1990 Sep 25;265(27):16343–16349. [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Lasnier F., Blin N., Baude B., Nahmias C., Strosberg A. D., Pairault J. Atypical beta-adrenergic receptor in 3T3-F442A adipocytes. Pharmacological and molecular relationship with the human beta 3-adrenergic receptor. J Biol Chem. 1991 Oct 25;266(30):20329–20336. [PubMed] [Google Scholar]
- Giacobino J. P. Subcellular fractionation of brown adipose tissue. J Supramol Struct. 1979;11(4):445–449. doi: 10.1002/jss.400110403. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N. Differential adrenergic regulation of beta 1- and beta 3-adrenoreceptor messenger ribonucleic acids in adipose tissues. Endocrinology. 1992 Jan;130(1):109–114. doi: 10.1210/endo.130.1.1309320. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Gawler D. J., Milligan G., Wilson A. Multiple defects occur in the guanine nucleotide regulatory protein system in liver plasma membranes of obese (fa/fa) but not lean (Fa/Fa) Zucker rats: loss of functional Gi and abnormal Gs function. Cell Signal. 1989;1(1):9–22. doi: 10.1016/0898-6568(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. Gi-2 is at the centre of an active phosphorylation/dephosphorylation cycle in hepatocytes: the fine-tuning of stimulatory and inhibitory inputs into adenylate cyclase in normal and diabetic states. Cell Signal. 1991;3(1):1–9. doi: 10.1016/0898-6568(91)90002-c. [DOI] [PubMed] [Google Scholar]
- Krief S., Bazin R., Dupuy F., Lavau M. Role of brown adipose tissue in glucose utilization in conscious pre-obese Zucker rats. Biochem J. 1989 Oct 1;263(1):305–308. doi: 10.1042/bj2630305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S., Bazin R. Genetic obesity: is the defect in the sympathetic nervous system? A review through developmental studies in the preobese Zucker rat. Proc Soc Exp Biol Med. 1991 Oct;198(1):528–538. doi: 10.3181/00379727-198-43286c. [DOI] [PubMed] [Google Scholar]
- Krief S., Fève B., Baude B., Zilberfarb V., Strosberg A. D., Pairault J., Emorine L. J. Transcriptional modulation by n-butyric acid of beta 1-, beta 2-, and beta 3-adrenergic receptor balance in 3T3-F442A adipocytes. J Biol Chem. 1994 Mar 4;269(9):6664–6670. [PubMed] [Google Scholar]
- Körtner G., Petrova O., Vogt S., Schmidt I. Sympathetically and nonsympathetically mediated onset of excess fat deposition in Zucker rats. Am J Physiol. 1994 Dec;267(6 Pt 1):E947–E953. doi: 10.1152/ajpendo.1994.267.6.E947. [DOI] [PubMed] [Google Scholar]
- Lafontan M., Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993 Jul;34(7):1057–1091. [PubMed] [Google Scholar]
- Lavau M., Bazin R. Inguinal fat pad weight plotted versus body weight as a method of genotype identification in 16-day-old Zucker rats. J Lipid Res. 1982 Aug;23(6):941–943. [PubMed] [Google Scholar]
- Levitzki A. From epinephrine to cyclic AMP. Science. 1988 Aug 12;241(4867):800–806. doi: 10.1126/science.2841758. [DOI] [PubMed] [Google Scholar]
- Marie V., Dupuy F., Bazin R. Decreased T4-to-T3 conversion in brown adipose tissue of Zucker fa/fa pups before the onset of obesity. Am J Physiol. 1992 Jul;263(1 Pt 1):E115–E120. doi: 10.1152/ajpendo.1992.263.1.E115. [DOI] [PubMed] [Google Scholar]
- Mitchell F. M., Griffiths S. L., Saggerson E. D., Houslay M. D., Knowler J. T., Milligan G. Guanine-nucleotide-binding proteins expressed in rat white adipose tissue. Identification of both mRNAs and proteins corresponding to Gi1, Gi2 and Gi3. Biochem J. 1989 Sep 1;262(2):403–408. doi: 10.1042/bj2620403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Fraser C. M., Giacobino J. P. Radioligand binding studies of the atypical beta 3-adrenergic receptor in rat brown adipose tissue using [3H]CGP 12177. FEBS Lett. 1992 Feb 24;298(2-3):162–164. doi: 10.1016/0014-5793(92)80046-j. [DOI] [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J. P., Fraser C. M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem. 1991 Dec 15;266(35):24053–24058. [PubMed] [Google Scholar]
- Nahmias C., Blin N., Elalouf J. M., Mattei M. G., Strosberg A. D., Emorine L. J. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991 Dec;10(12):3721–3727. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planche E., Joliff M., Bazin R. Energy expenditure and adipose tissue development in 2- to 8-day-old Zucker rats. Int J Obes. 1988;12(4):353–360. [PubMed] [Google Scholar]
- Ros M., Northup J. K., Malbon C. C. Adipocyte G-proteins and adenylate cyclase. Effects of adrenalectomy. Biochem J. 1989 Feb 1;257(3):737–744. doi: 10.1042/bj2570737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros M., Northup J. K., Malbon C. C. Steady-state levels of G-proteins and beta-adrenergic receptors in rat fat cells. Permissive effects of thyroid hormones. J Biol Chem. 1988 Mar 25;263(9):4362–4368. [PubMed] [Google Scholar]
- Strassheim D., Palmer T., Milligan G., Houslay M. D. Alterations in G-protein expression and the hormonal regulation of adenylate cyclase in the adipocytes of obese (fa/fa) Zucker rats. Biochem J. 1991 May 15;276(Pt 1):197–202. doi: 10.1042/bj2760197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett G. E., Bahary N., Friedman J. M., Leibel R. L. Rat obesity gene fatty (fa) maps to chromosome 5: evidence for homology with the mouse gene diabetes (db). Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7806–7809. doi: 10.1073/pnas.88.17.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]