Abstract
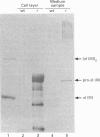
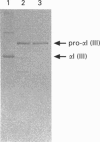
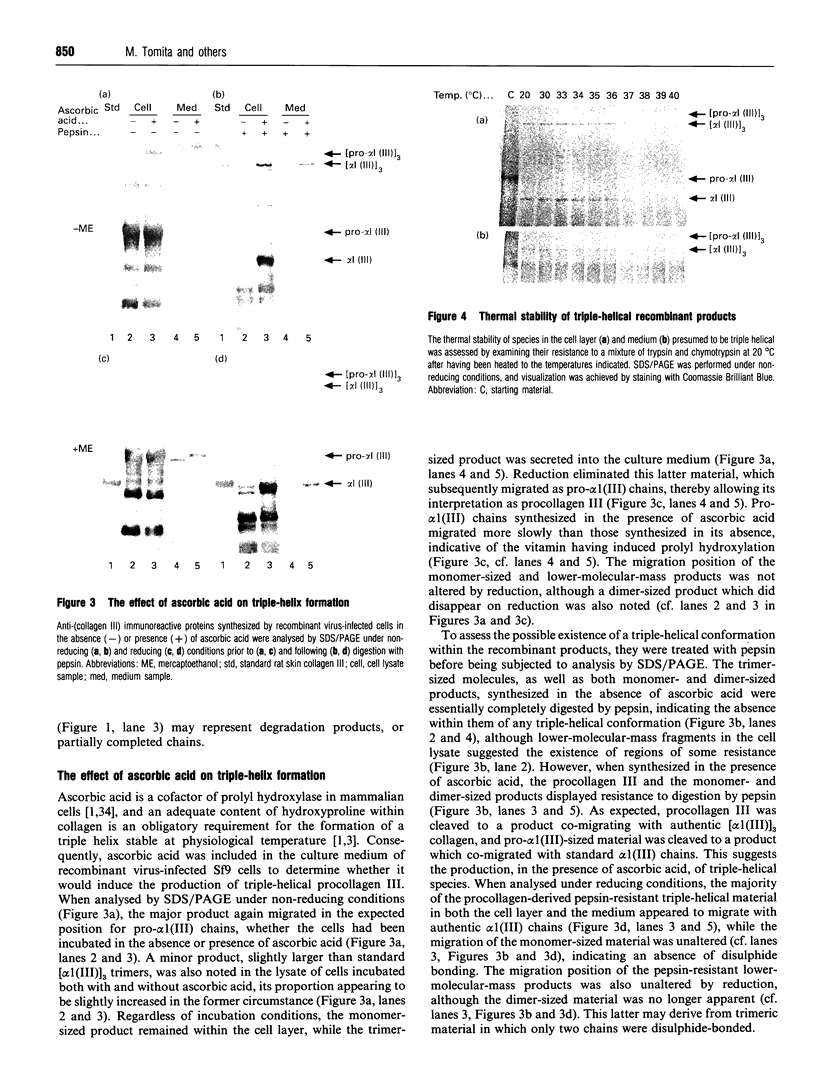
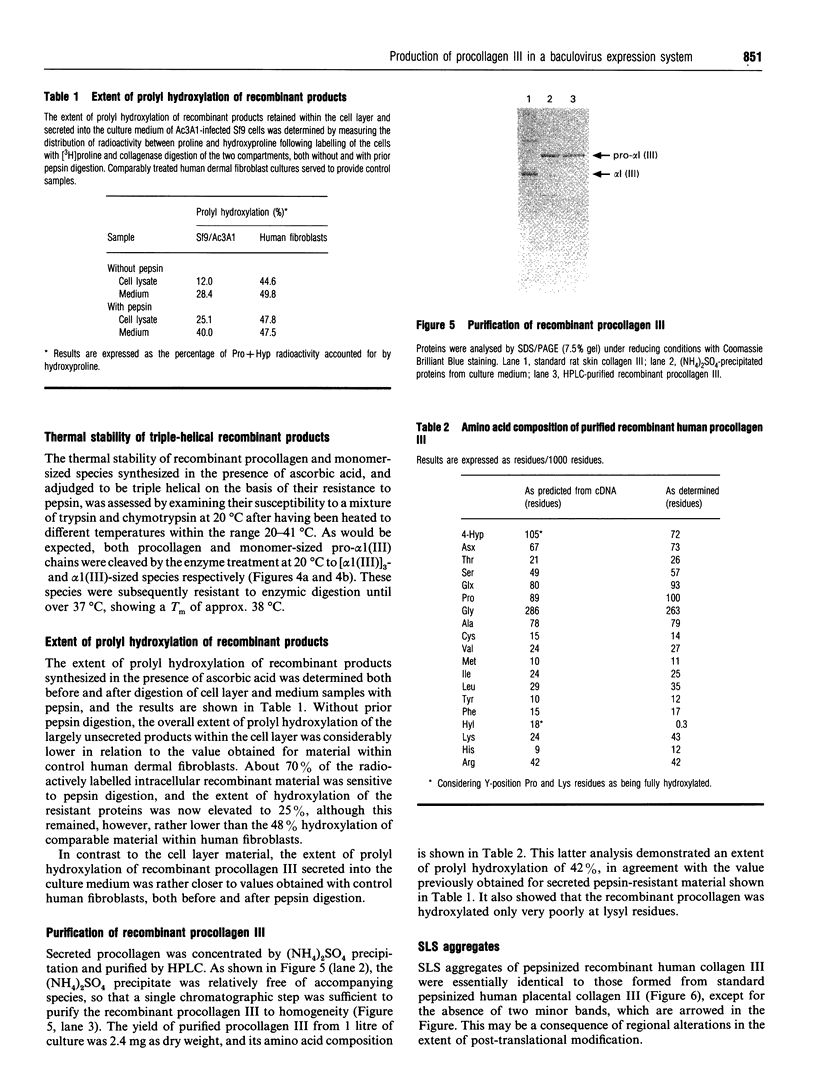
We have investigated the expression of human procollagen III by insect cells infected with a recombinant baculovirus carrying cDNA for the pro-alpha1(III) chain of type-III collagen. A high level of expression was obtained, and a small proportion of the heterologously expressed pro-alpha1(III) chains formed normally disulphide-bonded procollagen III, which was secreted into the culture medium. This species displayed a melting temperature (Tm) of approx. 38 degrees C as assessed by its resistance to digestion by a mixture of trypsin and chymotrypsin, slightly lower than that of 39.5 degrees C for procollagen III synthesized by cultured human dermal fibroblasts, and reflected a slight degree of under-hydroxylation of prolyl residues. This is possibly a consequence of the lower incubation temperature of insect cells, or of an insufficiency of prolyl hydroxylase activity within them. A significant proportion of the expressed chains formed trimeric molecules of similar thermal stability containing an apparently full-length triple-helical region, but were not disulphide-bonded and not secreted. In addition to providing a source of recombinant human procollagen III, the system promises to be useful in the study of procollagen chain association and subsequent folding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Hyland J., Smith C., Kivirikko K. I., Jimenez S. A., Prockop D. J. Expression of a human cartilage procollagen gene (COL2A1) in mouse 3T3 cells. J Biol Chem. 1991 Aug 5;266(22):14175–14178. [PubMed] [Google Scholar]
- Ala-Kokko L., Kontusaari S., Baldwin C. T., Kuivaniemi H., Prockop D. J. Structure of cDNA clones coding for the entire prepro alpha 1 (III) chain of human type III procollagen. Differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J. 1989 Jun 1;260(2):509–516. doi: 10.1042/bj2600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhurst D. E., Bailey A. J. Locust collagen: morphological and biochemical characterization. Eur J Biochem. 1980 Jan;103(1):75–83. doi: 10.1111/j.1432-1033.1980.tb04290.x. [DOI] [PubMed] [Google Scholar]
- Blumberg B., MacKrell A. J., Fessler J. H. Drosophila basement membrane procollagen alpha 1(IV). II. Complete cDNA sequence, genomic structure, and general implications for supramolecular assemblies. J Biol Chem. 1988 Dec 5;263(34):18328–18337. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cecchini J. P., Knibiehler B., Mirre C., Le Parco Y. Evidence for a type-IV-related collagen in Drosophila melanogaster. Evolutionary constancy of the carboxyl-terminal noncollagenous domain. Eur J Biochem. 1987 Jun 15;165(3):587–593. doi: 10.1111/j.1432-1033.1987.tb11480.x. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertala A., Sieron A. L., Ganguly A., Li S. W., Ala-Kokko L., Anumula K. R., Prockop D. J. Synthesis of recombinant human procollagen II in a stably transfected tumour cell line (HT1080). Biochem J. 1994 Feb 15;298(Pt 1):31–37. doi: 10.1042/bj2980031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertala A., Sieron A. L., Hojima Y., Ganguly A., Prockop D. J. Self-assembly into fibrils of collagen II by enzymic cleavage of recombinant procollagen II. Lag period, critical concentration, and morphology of fibrils differ from collagen I. J Biol Chem. 1994 Apr 15;269(15):11584–11589. [PubMed] [Google Scholar]
- Forster S. J., Freedman R. B. Catalysis by protein disulphide-isomerase of the assembly of trimeric procollagen from procollagen polypeptide chains. Biosci Rep. 1984 Mar;4(3):223–229. doi: 10.1007/BF01119657. [DOI] [PubMed] [Google Scholar]
- Geddis A. E., Prockop D. J. Expression of human COL1A1 gene in stably transfected HT1080 cells: the production of a thermostable homotrimer of type I collagen in a recombinant system. Matrix. 1993 Sep;13(5):399–405. doi: 10.1016/s0934-8832(11)80045-4. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Hoffman G. G., Lee B. S. High levels of expression of full length human pro-alpha 2(V) collagen cDNA in pro-alpha 2(V)-deficient hamster cells. J Biol Chem. 1989 Dec 5;264(34):20683–20687. [PubMed] [Google Scholar]
- Jimenez S. A., Yankowski R. Role of molecular conformation on secretion of chick tendon procollagen. J Biol Chem. 1978 Mar 10;253(5):1420–1426. [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J., Berg R. A. Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem. 1979 Apr 10;254(7):2234–2243. [PubMed] [Google Scholar]
- Kivirikko K. I. Collagens and their abnormalities in a wide spectrum of diseases. Ann Med. 1993 Apr;25(2):113–126. doi: 10.3109/07853899309164153. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R., Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989 Mar;3(5):1609–1617. [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Post-translational processing of procollagens. Ann N Y Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- Koivu J., Myllylä R. Interchain disulfide bond formation in types I and II procollagen. Evidence for a protein disulfide isomerase catalyzing bond formation. J Biol Chem. 1987 May 5;262(13):6159–6164. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. T., Smith B. D., Greenspan D. S. Construction of a full-length cDNA encoding human pro-alpha 2(I) collagen and its expression in pro-alpha 2(I)-deficient W8 rat cells. J Biol Chem. 1988 Sep 15;263(26):13414–13418. [PubMed] [Google Scholar]
- Lees J. F., Bulleid N. J. The role of cysteine residues in the folding and association of the COOH-terminal propeptide of types I and III procollagen. J Biol Chem. 1994 Sep 30;269(39):24354–24360. [PubMed] [Google Scholar]
- Lunstrum G. P., Bächinger H. P., Fessler L. I., Duncan K. G., Nelson R. E., Fessler J. H. Drosophila basement membrane procollagen IV. I. Protein characterization and distribution. J Biol Chem. 1988 Dec 5;263(34):18318–18327. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Middleton R. B., Bulleid N. J. Reconstitution of the folding pathway of collagen in a cell-free system: formation of correctly aligned and hydroxylated triple helices. Biochem J. 1993 Dec 1;296(Pt 2):511–517. doi: 10.1042/bj2960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Monson J. M. Assembly of procollagen mRNA translation products into pepsin-resistant structures. Coll Relat Res. 1983;3(1):1–12. doi: 10.1016/s0174-173x(83)80044-2. [DOI] [PubMed] [Google Scholar]
- Nakai A., Satoh M., Hirayoshi K., Nagata K. Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J Cell Biol. 1992 May;117(4):903–914. doi: 10.1083/jcb.117.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette L. A., Paglia L. M., Martin G. R. Characterization of the cell free translation products from types I and II procollagen mRNAs. Coll Relat Res. 1981 Jul;1(4):327–335. doi: 10.1016/s0174-173x(81)80009-x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce P. M., Blumberg A., Zurbrügg R. P., Zimmermann A., Colombo J. P., Steinmann B. Lethal osteogenesis imperfecta: abnormal collagen metabolism and biochemical characteristics of hypophosphatasia. Eur J Pediatr. 1988 Aug;147(6):626–631. doi: 10.1007/BF00442478. [DOI] [PubMed] [Google Scholar]
- Royce P. M., Danks D. M. Normal hydroxylation of proline in collagen synthesized by skin fibroblasts from a patient with prolidase deficiency. J Inherit Metab Dis. 1982;5(2):111–113. doi: 10.1007/BF01800003. [DOI] [PubMed] [Google Scholar]
- Royce P. M., Steinmann B., Vogel A., Steinhorst U., Kohlschuetter A. Brittle cornea syndrome: an heritable connective tissue disorder distinct from Ehlers-Danlos syndrome type VI and fragilitas oculi, with spontaneous perforations of the eye, blue sclerae, red hair, and normal collagen lysyl hydroxylation. Eur J Pediatr. 1990 Apr;149(7):465–469. doi: 10.1007/BF01959396. [DOI] [PubMed] [Google Scholar]
- Sauk J. J., Smith T., Norris K., Ferreira L. Hsp47 and the translation-translocation machinery cooperate in the production of alpha 1(I) chains of type I procollagen. J Biol Chem. 1994 Feb 11;269(6):3941–3946. [PubMed] [Google Scholar]
- Schnieke A., Dziadek M., Bateman J., Mascara T., Harbers K., Gelinas R., Jaenisch R. Introduction of the human pro alpha 1(I) collagen gene into pro alpha 1(I)-deficient Mov-13 mouse cells leads to formation of functional mouse-human hybrid type I collagen. Proc Natl Acad Sci U S A. 1987 Feb;84(3):764–768. doi: 10.1073/pnas.84.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M. Interstitial collagen polymorphism in rat liver with CCl4-induced cirrhosis. Biochim Biophys Acta. 1980 May 22;629(3):490–498. doi: 10.1016/0304-4165(80)90154-3. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Gugler E., Gitzelmann R., Steinmann B. Ehlers-Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem. 1988 May 5;263(13):6226–6232. [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier D. C., Thomas D. Y., Khouri H. E., Laliberté F., Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991 Feb 15;98(2):177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- Wiedemann H., Chung E., Fujii T., Miller E. J., Kühn K. Comparative electron-microscope studies on type-III and type-I collagens. Eur J Biochem. 1975 Feb 21;51(2):363–368. doi: 10.1111/j.1432-1033.1975.tb03936.x. [DOI] [PubMed] [Google Scholar]