Abstract
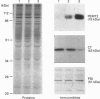
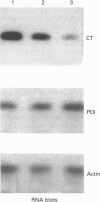
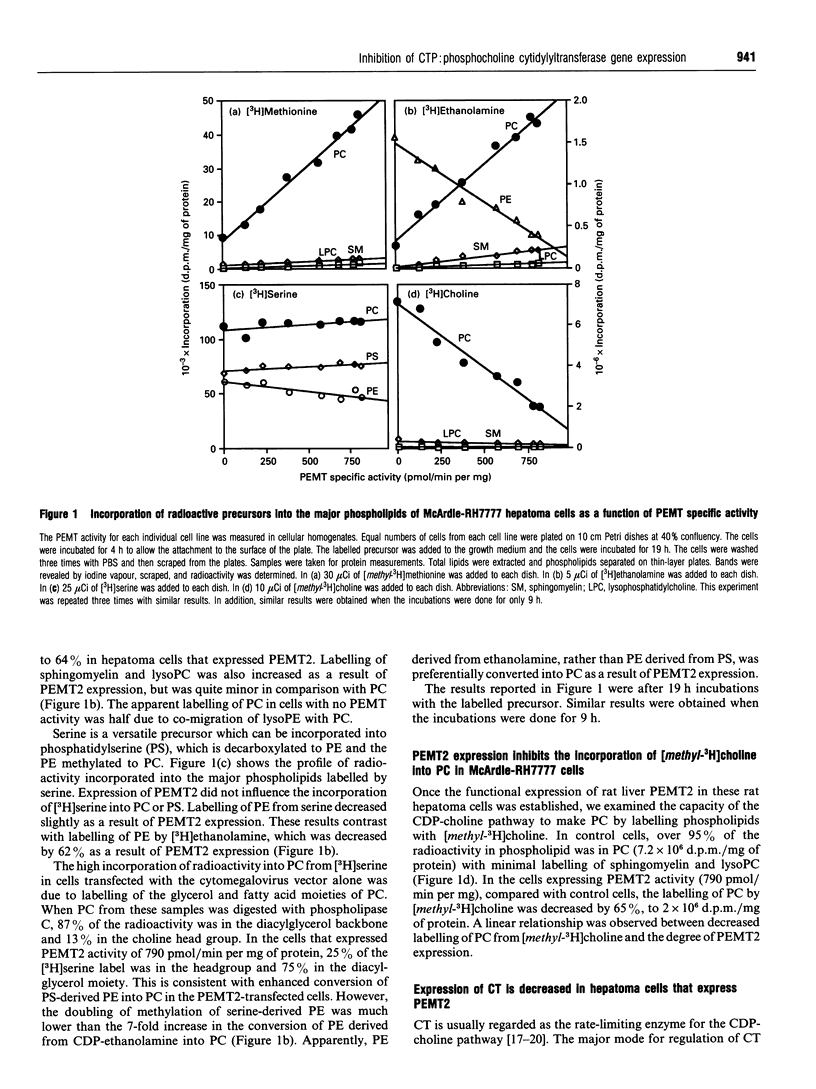
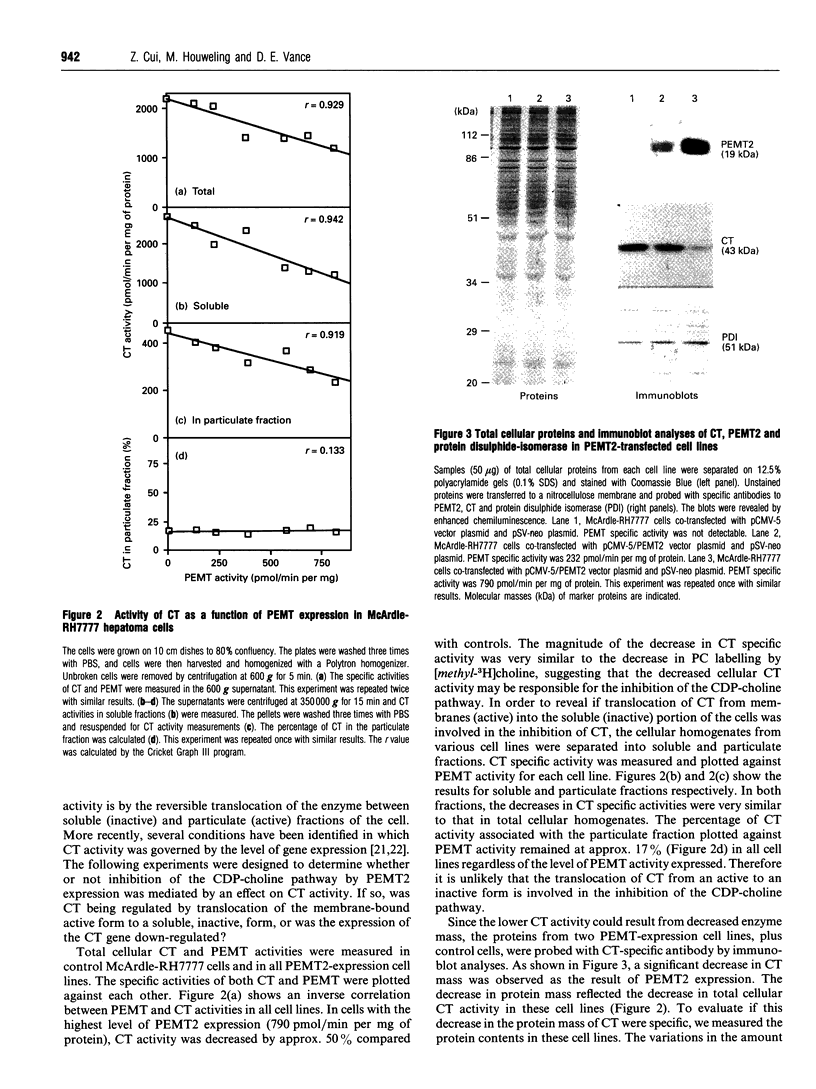
Phosphatidylethanolamine N-methyltransferase-2 (PEMT2) of rat liver was expressed in McArdle-RH7777 rat hepatoma cells, which lack endogenous PEMT activity. Expression of the enzyme was confirmed by assay of PEMT activity and immunoblotting. There was no change in the amount of phosphatidylcholine in the transfected cells [Cu, Houweling and Vance (1994) J. Biol. Chem. 269, 24531-24533], even though the expression of PEMT2 caused an increased incorporation of [methyl-3H]methionine and [3H]ethanolamine into phosphatidylcholine. In contrast, [3H]serine incorporation into phosphatidylcholine was only marginally enhanced by PEMT2 expression. Incorporation of [methyl-3H]choline into phosphatidylcholine was decreased by greater than 60%, suggesting that the CDP-choline pathway was inhibited as a result of PEMT2 expression. CTP:phosphocholine cytidylyltransferase (CT) activities in transfected cell lines were decreased in proportion to the level of expression of PEMT2. Immunoblot analyses showed a decrease in CT mass as a function of PEMT2 expression. In contrast, there was no change in the mass of protein disulphide-isomerase or the relative amounts of most proteins expressed in the PEMT2-transfected, compared with control, cells. Similarly, the expression of CT mRNA was decreased in PEMT2-expressing cells, whereas the mRNAs for protein disulphide-isomerase and actin were unchanged. When cell growth was slowed by incubating McArdle-RH7777 cells at 25 degrees C, compared with 37 degrees C, there was no difference in the specific activity of the CT. These results argue that PEMT2 expression down-regulates the CDP-choline pathway by decreasing the expression of the gene for the CT. The decreased activity of the CDP-choline pathway might contribute to the slower rate of cell division in PEMT2-transfected hepatoma cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriamampandry C., Freysz L., Kanfer J. N., Dreyfus H., Massarelli R. Conversion of ethanolamine, monomethylethanolamine and dimethylethanolamine to choline-containing compounds by neurons in culture and by the rat brain. Biochem J. 1989 Dec 1;264(2):555–562. doi: 10.1042/bj2640555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audubert F., Pelech S. L., Vance D. E. Fatty acids inhibit N-methylation of phosphatidylethanolamine in rat hepatocytes and liver microsomes. Biochim Biophys Acta. 1984 Mar 7;792(3):348–357. doi: 10.1016/0005-2760(84)90203-0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Houweling M., Vance D. E. Suppression of rat hepatoma cell growth by expression of phosphatidylethanolamine N-methyltransferase-2. J Biol Chem. 1994 Oct 7;269(40):24531–24533. [PubMed] [Google Scholar]
- Cui Z., Vance J. E., Chen M. H., Voelker D. R., Vance D. E. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J Biol Chem. 1993 Aug 5;268(22):16655–16663. [PubMed] [Google Scholar]
- EAGLE H. The minimum vitamin requirements of the L and HeLa cells in tissue culture, the production of specific vitamin deficiencies, and their cure. J Exp Med. 1955 Nov 1;102(5):595–600. doi: 10.1084/jem.102.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Wermuth M. M., Raetz C. R. Thermolabile CDP-choline synthetase in an animal cell mutant defective in lecithin formation. J Biol Chem. 1981 Jul 25;256(14):7388–7393. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hatch G. M., Jamil H., Utal A. K., Vance D. E. On the mechanism of the okadaic acid-induced inhibition of phosphatidylcholine biosynthesis in isolated rat hepatocytes. J Biol Chem. 1992 Aug 5;267(22):15751–15758. [PubMed] [Google Scholar]
- Houweling M., Cui Z., Vance D. E. Expression of phosphatidylethanolamine N-methyltransferase-2 cannot compensate for an impaired CDP-choline pathway in mutant Chinese hamster ovary cells. J Biol Chem. 1995 Jul 7;270(27):16277–16282. doi: 10.1074/jbc.270.27.16277. [DOI] [PubMed] [Google Scholar]
- Houweling M., Jamil H., Hatch G. M., Vance D. E. Dephosphorylation of CTP-phosphocholine cytidylyltransferase is not required for binding to membranes. J Biol Chem. 1994 Mar 11;269(10):7544–7551. [PubMed] [Google Scholar]
- Houweling M., Tijburg L. B., Jamil H., Vance D. E., Nyathi C. B., Vaartjes W. J., van Golde L. M. Phosphatidylcholine metabolism in rat liver after partial hepatectomy. Evidence for increased activity and amount of CTP:phosphocholine cytidylyltransferase. Biochem J. 1991 Sep 1;278(Pt 2):347–351. doi: 10.1042/bj2780347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling M., Tijburg L. B., Vaartjes W. J., Batenburg J. J., Kalmar G. B., Cornell R. B., Van Golde L. M. Evidence that CTP:choline-phosphate cytidylyltransferase is regulated at a pretranslational level in rat liver after partial hepatectomy. Eur J Biochem. 1993 Jun 15;214(3):927–933. doi: 10.1111/j.1432-1033.1993.tb17996.x. [DOI] [PubMed] [Google Scholar]
- Jackowski S. Coordination of membrane phospholipid synthesis with the cell cycle. J Biol Chem. 1994 Feb 4;269(5):3858–3867. [PubMed] [Google Scholar]
- Jamil H., Utal A. K., Vance D. E. Evidence that cyclic AMP-induced inhibition of phosphatidylcholine biosynthesis is caused by a decrease in cellular diacylglycerol levels in cultured rat hepatocytes. J Biol Chem. 1992 Jan 25;267(3):1752–1760. [PubMed] [Google Scholar]
- Kalmar G. B., Kay R. J., Lachance A., Aebersold R., Cornell R. B. Cloning and expression of rat liver CTP: phosphocholine cytidylyltransferase: an amphipathic protein that controls phosphatidylcholine synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6029–6033. doi: 10.1073/pnas.87.16.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C. Regulation of phosphatidylcholine biosynthesis. Prog Lipid Res. 1990;29(2):87–105. doi: 10.1016/0163-7827(90)90010-i. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDonald J. I., Kent C. Identification of phosphorylation sites in rat liver CTP: phosphocholine cytidylyltransferase. J Biol Chem. 1994 Apr 8;269(14):10529–10537. [PubMed] [Google Scholar]
- Mudd S. H., Datko A. H. Synthesis of methylated ethanolamine moieties: regulation by choline in lemna. Plant Physiol. 1989 May;90(1):296–305. doi: 10.1104/pp.90.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Pritchard P. H., Sommerman E. F., Percival-Smith A., Vance D. E. Glucagon inhibits phosphatidylcholine biosynthesis via the CDP-choline and transmethylation pathways in cultured rat hepatocytes. Can J Biochem Cell Biol. 1984 Apr;62(4):196–202. doi: 10.1139/o84-028. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of rat liver cytosolic CTP: phosphocholine cytidylyltransferase by phosphorylation and dephosphorylation. J Biol Chem. 1982 Dec 10;257(23):14198–14202. [PubMed] [Google Scholar]
- Pritchard P. H., Chiang P. K., Cantoni G. L., Vance D. E. Inhibition of phosphatidylethanolamine N-methylation by 3-deazaadenosine stimulates the synthesis of phosphatidylcholine via the CDP-choline pathway. J Biol Chem. 1982 Jun 10;257(11):6362–6367. [PubMed] [Google Scholar]
- Pritchard P. H., Pelech S. L., Vance D. E. Analogues of cyclic AMP inhibit phosphatidylethanolamine N-methylation by cultured rat hepatocytes. Biochim Biophys Acta. 1981 Nov 23;666(2):301–306. doi: 10.1016/0005-2760(81)90122-3. [DOI] [PubMed] [Google Scholar]
- Prud'homme M. P., Moore T. S. Phosphatidylcholine synthesis in castor bean endosperm : free bases as intermediates. Plant Physiol. 1992 Nov;100(3):1527–1535. doi: 10.1104/pp.100.3.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway N. D., Vance D. E. Phosphatidylethanolamine N-methyltransferase from rat liver. Methods Enzymol. 1992;209:366–374. doi: 10.1016/0076-6879(92)09045-5. [DOI] [PubMed] [Google Scholar]
- Ridgway N. D., Yao Z., Vance D. E. Phosphatidylethanolamine levels and regulation of phosphatidylethanolamine N-methyltransferase. J Biol Chem. 1989 Jan 15;264(2):1203–1207. [PubMed] [Google Scholar]
- Rutherford M. S., Rock C. O., Jenkins N. A., Gilbert D. J., Tessner T. G., Copeland N. G., Jackowski S. The gene for murine CTP:phosphocholine cytidylyltransferase (Ctpct) is located on mouse chromosome 16. Genomics. 1993 Dec;18(3):698–701. doi: 10.1016/s0888-7543(05)80377-5. [DOI] [PubMed] [Google Scholar]
- Schneider W. J., Vance D. E. Effect of choline deficiency on the enzymes that synthesize phosphatidylcholine and phosphatidylethanolamine in rat liver. Eur J Biochem. 1978 Apr;85(1):181–187. doi: 10.1111/j.1432-1033.1978.tb12226.x. [DOI] [PubMed] [Google Scholar]
- Skinner H. B., McGee T. P., McMaster C. R., Fry M. R., Bell R. M., Bankaitis V. A. The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):112–116. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Summers P. S., Weretilnyk E. A. Choline Synthesis in Spinach in Relation to Salt Stress. Plant Physiol. 1993 Dec;103(4):1269–1276. doi: 10.1104/pp.103.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer T. D., Kent C. Expression of wild-type and mutant rat liver CTP: phosphocholine cytidylyltransferase in a cytidylyltransferase-deficient Chinese hamster ovary cell line. Arch Biochem Biophys. 1994 May 15;311(1):107–116. doi: 10.1006/abbi.1994.1215. [DOI] [PubMed] [Google Scholar]
- Tercé F., Brun H., Vance D. E. Requirement of phosphatidylcholine for normal progression through the cell cycle in C3H/10T1/2 fibroblasts. J Lipid Res. 1994 Dec;35(12):2130–2142. [PubMed] [Google Scholar]
- Tessner T. G., Rock C. O., Kalmar G. B., Cornell R. B., Jackowski S. Colony-stimulating factor 1 regulates CTP: phosphocholine cytidylyltransferase mRNA levels. J Biol Chem. 1991 Sep 5;266(25):16261–16264. [PubMed] [Google Scholar]
- Tijburg L. B., Geelen M. J., van Golde L. M. Regulation of the biosynthesis of triacylglycerol, phosphatidylcholine and phosphatidylethanolamine in the liver. Biochim Biophys Acta. 1989 Jul 17;1004(1):1–19. doi: 10.1016/0005-2760(89)90206-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchère H., Record M., Tercé F., Chap H. Phosphatidylcholine cycle and regulation of phosphatidylcholine biosynthesis by enzyme translocation. Biochim Biophys Acta. 1994 May 13;1212(2):137–151. doi: 10.1016/0005-2760(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Vance D. E. Boehringer Mannheim Award lecture. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem Cell Biol. 1990 Oct;68(10):1151–1165. doi: 10.1139/o90-172. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Pelech S. D., Choy P. C. CTP: phosphocholine cytidylyltransferase from rat liver. Methods Enzymol. 1981;71(Pt 100):576–581. doi: 10.1016/0076-6879(81)71070-x. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Ridgway N. D. The methylation of phosphatidylethanolamine. Prog Lipid Res. 1988;27(1):61–79. doi: 10.1016/0163-7827(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Vance J. E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990 May 5;265(13):7248–7256. [PubMed] [Google Scholar]
- Weinhold P. A., Charles L., Feldman D. A. Regulation of CTP: phosphocholine cytidylyltransferase in HepG2 cells: effect of choline depletion on phosphorylation, translocation and phosphatidylcholine levels. Biochim Biophys Acta. 1994 Jan 20;1210(3):335–347. doi: 10.1016/0005-2760(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Niwa A., Yasumura Y. Continuous growth and phosphatidylcholine synthesis of rat hepatoma cells in choline-deprived chemically defined medium. J Cell Physiol. 1985 Oct;125(1):91–97. doi: 10.1002/jcp.1041250112. [DOI] [PubMed] [Google Scholar]