Abstract
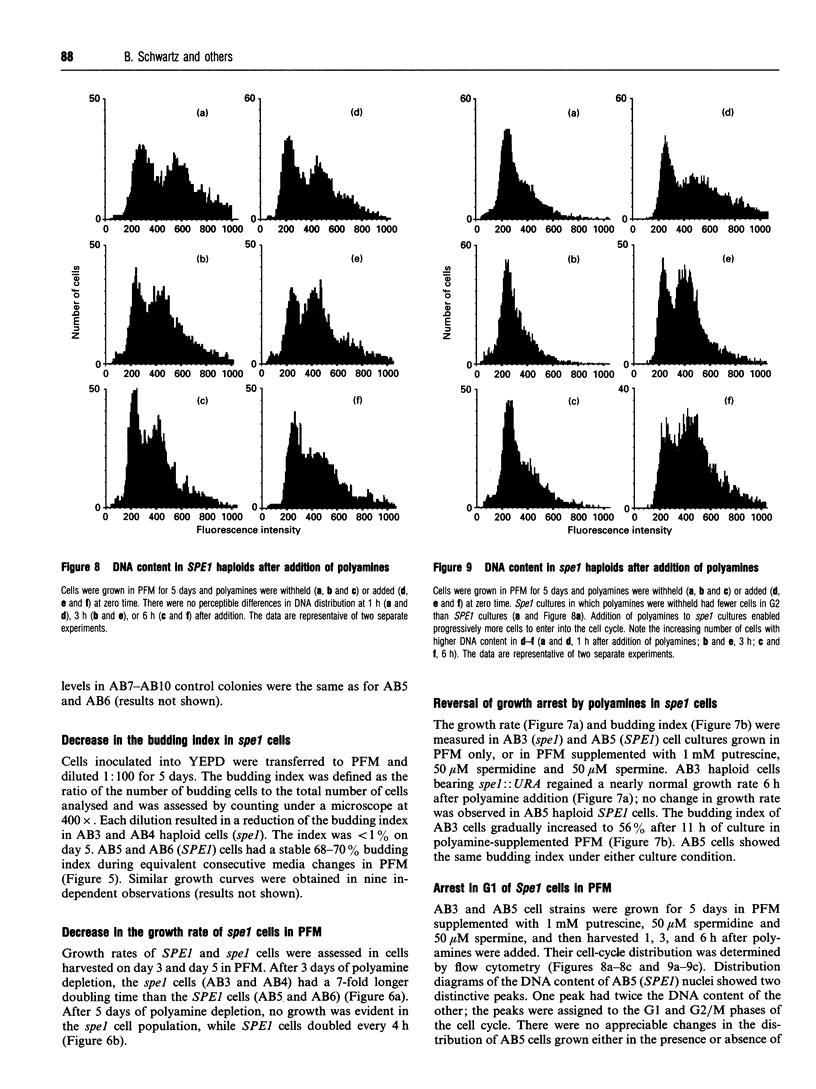
Ornithine decarboxylase (ODC) is a rate-determining enzyme of the polyamine-biosynthetic pathway. We sought to produce cells with impaired ODC function in order to study the biological functions of polyamines. Saccharomyces cerevisiae strains were obtained by one-step gene replacement of a 900 bp fragment of the yeast ODC gene (SPE1) with the yeast URA3 gene. Spores derived from SPE1/spe1 cells germinated at reduced efficiency relative to SPE1/SPE1. Sustained growth of spe1 haploid mutants in polyamine-free medium led to intracellular polyamine depletion, reduction in budding index, G1 arrest and cessation of growth, and cells that were large and misshapen. All of these effects were completely reversed by adding polyamines to the medium, even after 5 days of polyamine starvation. A diploid yeast strain bearing two copies of disrupted spe1 lost heterozygosity at the mating-type locus more often when grown in the absence of polyamines than when grown in their presence, indicating that polyamine deficiency leads to either chromosome loss or to mitotic recombination.
Full text
PDF

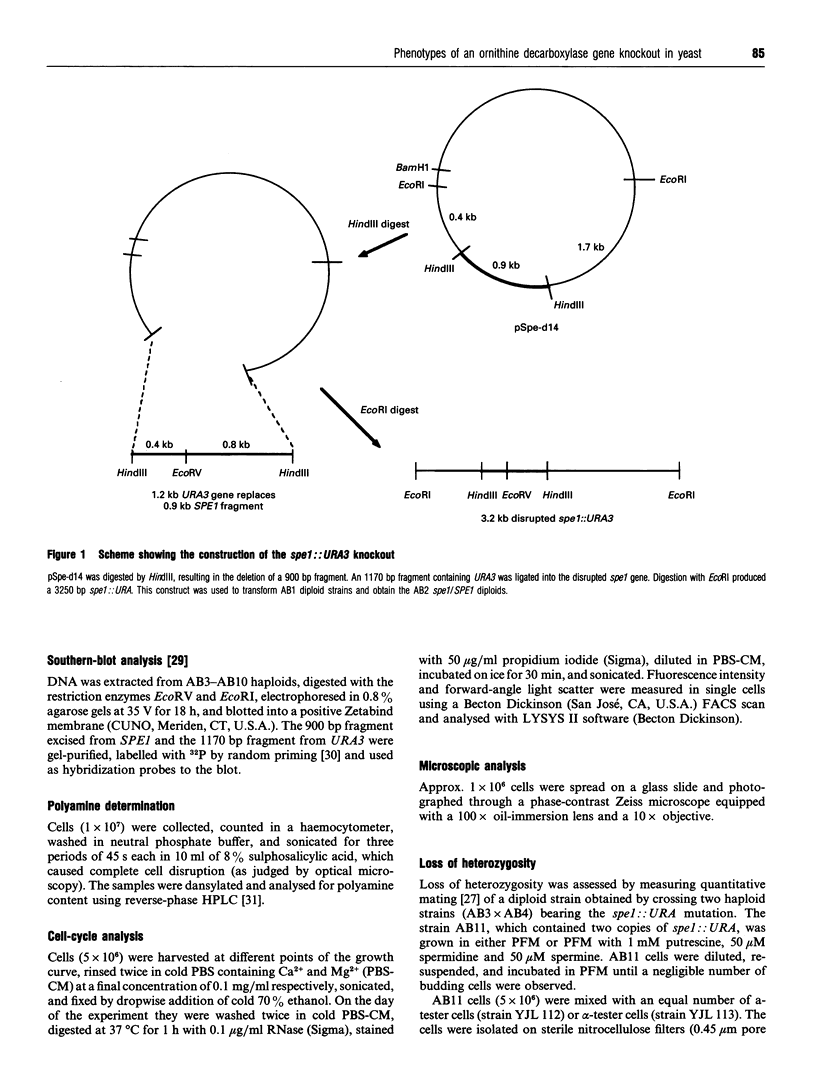
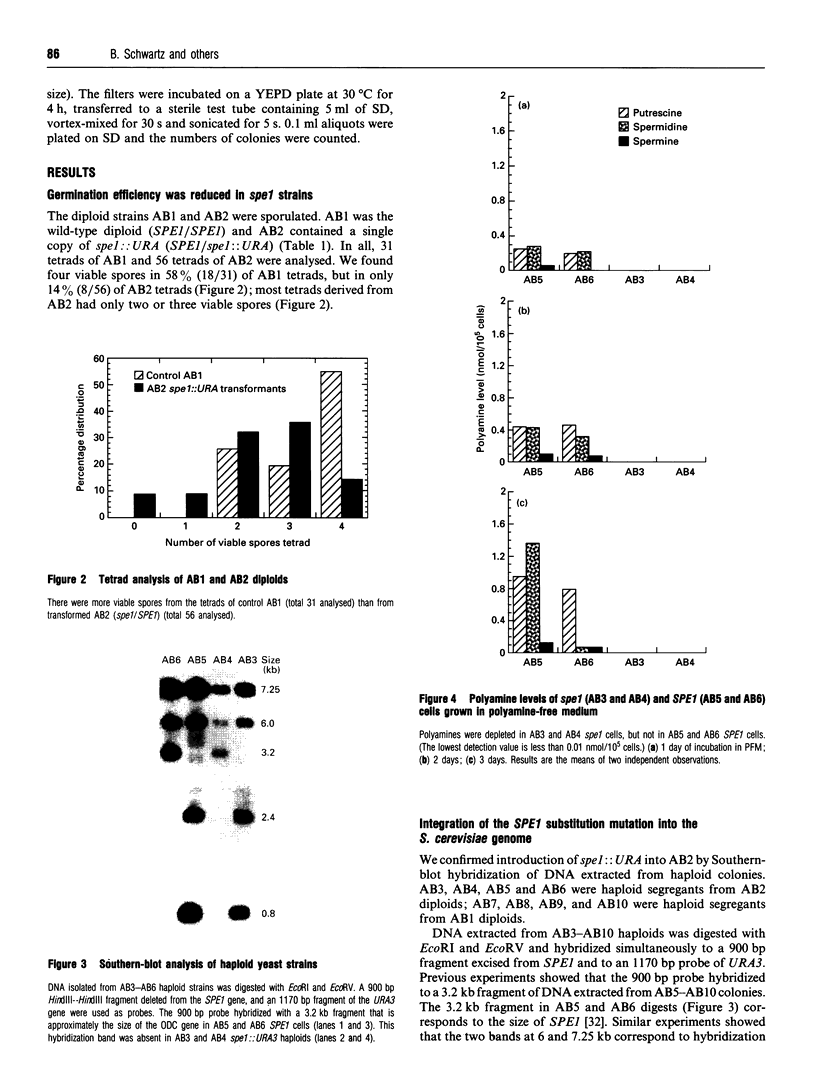
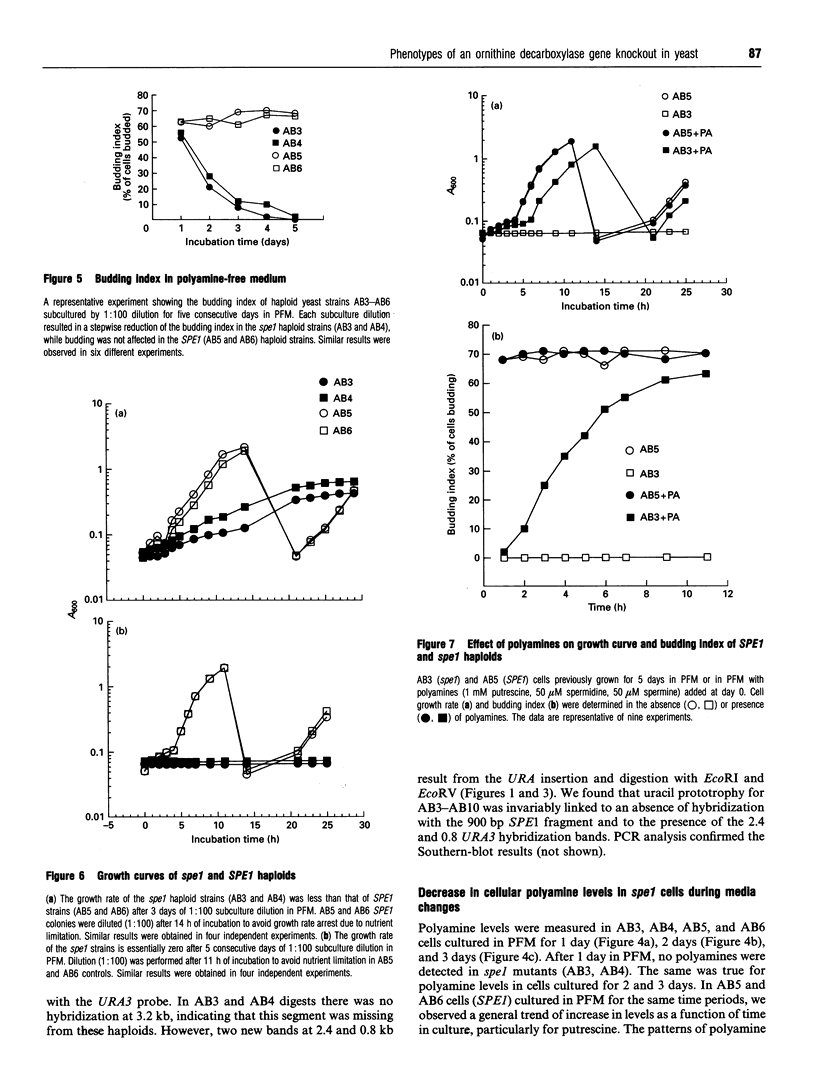


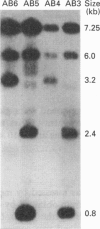
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auvinen M., Paasinen A., Andersson L. C., Hölttä E. Ornithine decarboxylase activity is critical for cell transformation. Nature. 1992 Nov 26;360(6402):355–358. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- Balasundaram D., Dinman J. D., Wickner R. B., Tabor C. W., Tabor H. Spermidine deficiency increases +1 ribosomal frameshifting efficiency and inhibits Ty1 retrotransposition in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):172–176. doi: 10.1073/pnas.91.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram D., Tabor C. W., Tabor H. Spermidine or spermine is essential for the aerobic growth of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5872–5876. doi: 10.1073/pnas.88.13.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu H. S., Pellarin M., Feuerstein B. G., Deen D. F., Marton L. J. Effect on N1,N14-bis-(ethyl)-homospermine (BE-4-4-4) on the growth of U-251 MG and SF-188 human brain tumor cells. Int J Cancer. 1991 Jul 30;48(6):873–878. doi: 10.1002/ijc.2910480614. [DOI] [PubMed] [Google Scholar]
- Basu H. S., Pellarin M., Feuerstein B. G., Shirahata A., Samejima K., Deen D. F., Marton L. J. Interaction of a polyamine analogue, 1,19-bis-(ethylamino)-5,10,15- triazanonadecane (BE-4-4-4-4), with DNA and effect on growth, survival, and polyamine levels in seven human brain tumor cell lines. Cancer Res. 1993 Sep 1;53(17):3948–3955. [PubMed] [Google Scholar]
- Basu H. S., Schwietert H. C., Feuerstein B. G., Marton L. J. Effects of variation in the structure of spermine on the association with DNA and the induction of DNA conformational changes. Biochem J. 1990 Jul 15;269(2):329–334. doi: 10.1042/bj2690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu H. S., Sturkenboom M. C., Delcros J. G., Csokan P. P., Szollosi J., Feuerstein B. G., Marton L. J. Effect of polyamine depletion on chromatin structure in U-87 MG human brain tumour cells. Biochem J. 1992 Mar 15;282(Pt 3):723–727. doi: 10.1042/bj2820723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billett M. A., Hall T. J. Cations and the accessibility of chromatin to nucleases. Nucleic Acids Res. 1979 Jun 25;6(8):2929–2945. doi: 10.1093/nar/6.8.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Regulatory mutations affecting ornithine decarboxylase activity in Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):791–799. doi: 10.1128/jb.142.3.791-799.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Molecular mechanics of the interactions of spermine with DNA: DNA bending as a result of ligand binding. Nucleic Acids Res. 1990 Mar 11;18(5):1271–1282. doi: 10.1093/nar/18.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi W. A., Sypherd P. S. The gene and the primary structure of ornithine decarboxylase from Saccharomyces cerevisiae. J Biol Chem. 1987 Jul 25;262(21):10127–10133. [PubMed] [Google Scholar]
- Jain A., Tyagi A. K. Role of polyamines in the synthesis of RNA in mycobacteria. Mol Cell Biochem. 1987 Nov;78(1):3–8. doi: 10.1007/BF00224418. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kabra P. M., Lee H. K., Lubich W. P., Marton L. J. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr. 1986 Jul 11;380(1):19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- Knuutila S., Pohjanpelto P. Polyamine starvation causes parallel increase in nuclear and chromosomal aberrations in a polyamine-dependent strain of CHO. Exp Cell Res. 1983 Apr 15;145(1):222–226. doi: 10.1016/s0014-4827(83)80024-x. [DOI] [PubMed] [Google Scholar]
- Koza R. A., Herbst E. J. Deficiencies in DNA replication and cell-cycle progression in polyamine-depleted HeLa cells. Biochem J. 1992 Jan 1;281(Pt 1):87–93. doi: 10.1042/bj2810087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton L. J., Pegg A. E., Morris D. R. Directions for polyamine research. J Cell Biochem. 1991 Jan;45(1):7–8. doi: 10.1002/jcb.240450105. [DOI] [PubMed] [Google Scholar]
- Palvimo J., Pohjanpelto P., Linnala-Kankkunen A., Mäenpä P. H. Alterations in amounts and covalent modifications of low-molecular-weight chromosomal proteins in Chinese hamster ovary cells during polyamine depletion. Biochim Biophys Acta. 1987 Jun 6;909(1):21–29. doi: 10.1016/0167-4781(87)90042-x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P., Knuutila S. Induction of major chromosome aberrations in Chinese hamster ovary cells by alpha-difluoromethylornithine. Cancer Res. 1984 Oct;44(10):4535–4539. [PubMed] [Google Scholar]
- Shalitin C., Vishlizky A. Spermidine inhibits degradation of yeast chromatin. Biochim Biophys Acta. 1984 Jul 18;782(3):328–330. doi: 10.1016/0167-4781(84)90069-1. [DOI] [PubMed] [Google Scholar]
- Snyder R. D. Polyamine depletion is associated with altered chromatin structure in HeLa cells. Biochem J. 1989 Jun 15;260(3):697–704. doi: 10.1042/bj2600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]