Abstract
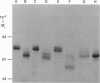
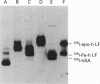
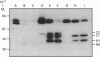
We studied the role of N-glycosylation of human lactoferrin (hLF) with respect to properties that are relevant to its antibacterial and anti-inflammatory activities. A human kidney-derived 293(S) cell line that constitutively expresses recombinant hLF (rhLF) was produced. The reactivity towards various antibodies of rhLF that had been expressed in the absence or presence of tunicamycin (which blocks N-linked glycosylation) did not differ from that of natural (human milk-derived) hLF. Cation-exchange chromatography and N-terminal protein sequencing showed identical cationic properties and an intact N-terminal sequence for rhLF and natural hLF. SDS/PAGE of rhLF expressed in the presence of tunicamycin revealed a protein with the same M(r) as that of enzymically deglycosylated natural hLF. Both glycosylated and unglycosylated rhLF appeared to be completely saturated with iron. The affinity of natural hLF, glycosylated and non-glycosylated rhLF for both human lysozyme (Kd 4.5 x 10(-8) M) and bacterial lipopolysaccharide did not differ. SDS/PAGE of hLF species subjected to trypsin indicated that unglycosylated rhLF was much more susceptible to degradation. Furthermore, this analysis suggests that N-glycosylation heterogeneity in natural hLF and rhLF resides in the C-lobe. Thus our results provide no argument for differential antibacterial and/or anti-inflammatory activity of natural and (glycosylated) rhLF and suggest that a major function of glycosylation in hLF is to protect it against proteolysis.
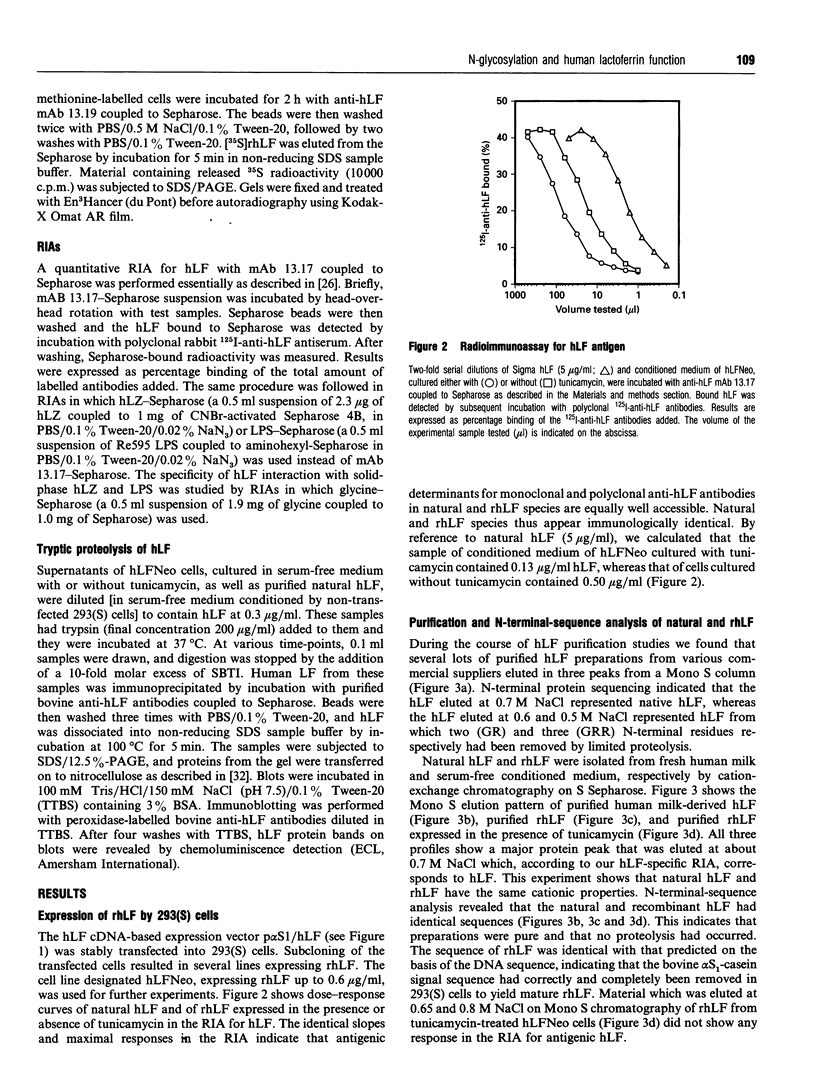
Full text
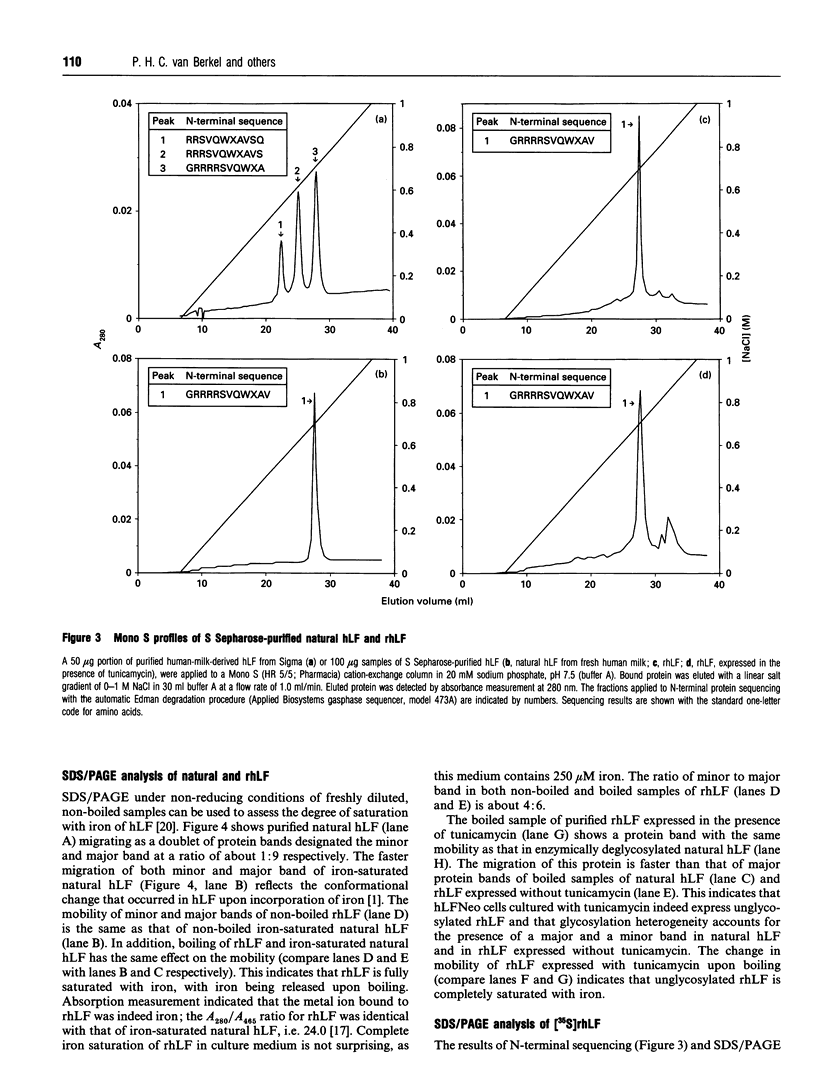
PDF

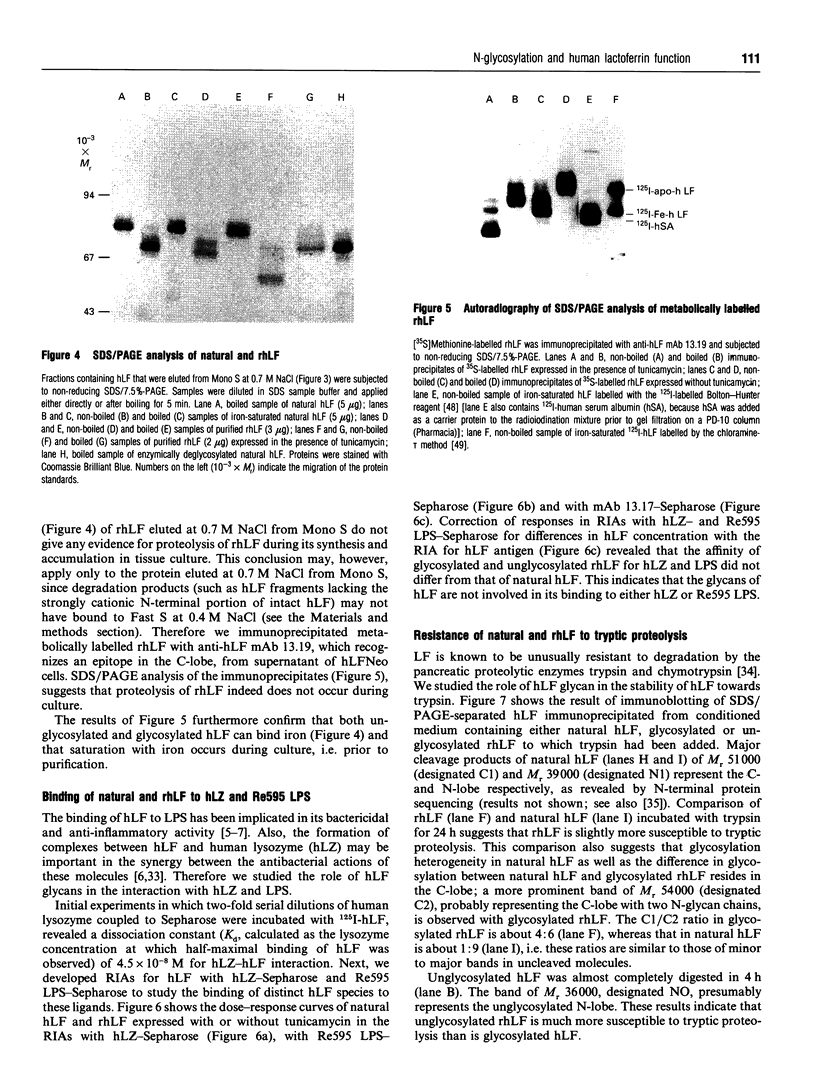
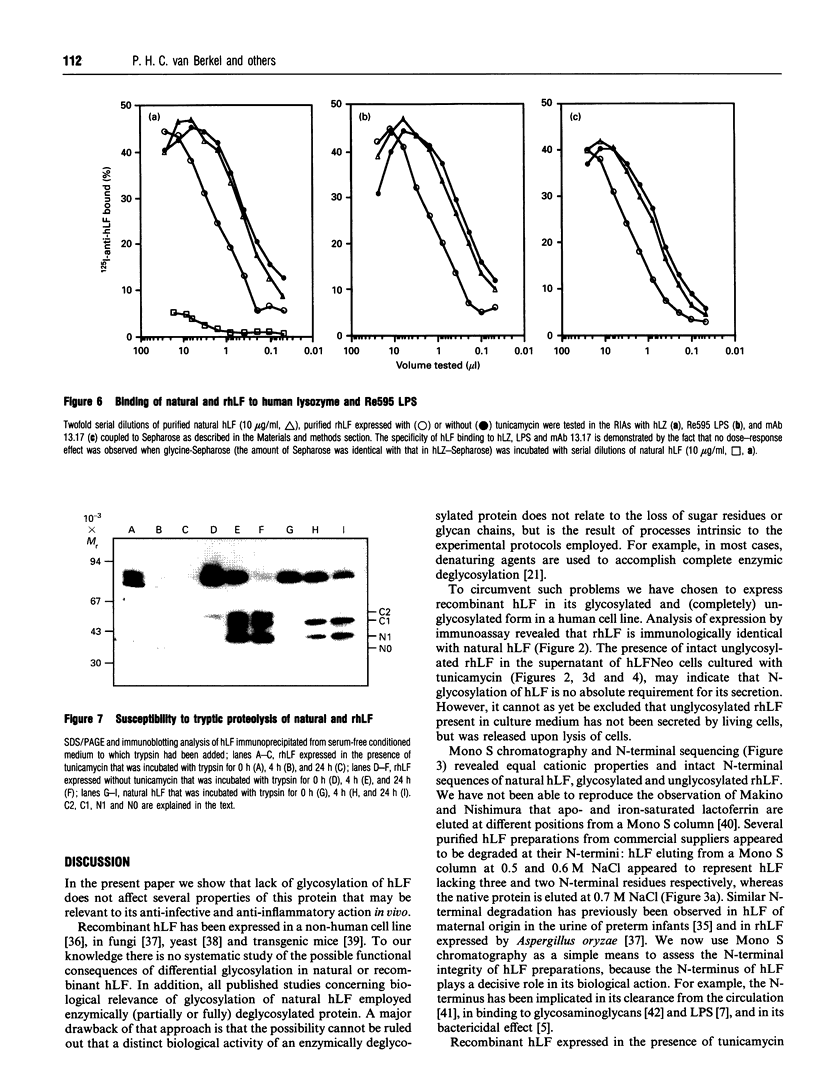


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Appelmelk B. J., An Y. Q., Geerts M., Thijs B. G., de Boer H. A., MacLaren D. M., de Graaff J., Nuijens J. H. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994 Jun;62(6):2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines R. D., Brock J. H. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim Biophys Acta. 1983 Sep 13;759(3):229–235. doi: 10.1016/0304-4165(83)90317-3. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Hassett D. J., Rosen G. M., Hamill D. R., Cohen M. S. Neutrophil degranulation inhibits potential hydroxyl-radical formation. Relative impact of myeloperoxidase and lactoferrin release on hydroxyl-radical production by iron-supplemented neutrophils assessed by spin-trapping techniques. Biochem J. 1989 Dec 1;264(2):447–455. doi: 10.1042/bj2640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T., Huang M., Gorman C., Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol. 1991 Jun;11(6):3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddeville B., Strecker G., Wieruszeski J. M., Vliegenthart J. F., van Halbeek H., Peter-Katalinić J., Egge H., Spik G. Heterogeneity of bovine lactotransferrin glycans. Characterization of alpha-D-Galp-(1-->3)-beta-D-Gal- and alpha-NeuAc-(2-->6)-beta-D-GalpNAc-(1-->4)- beta-D-GlcNAc-substituted N-linked glycans. Carbohydr Res. 1992 Dec 15;236:145–164. doi: 10.1016/0008-6215(92)85013-p. [DOI] [PubMed] [Google Scholar]
- Cox T. M., Mazurier J., Spik G., Montreuil J., Peters T. J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979 Nov 15;588(1):120–128. doi: 10.1016/0304-4165(79)90377-5. [DOI] [PubMed] [Google Scholar]
- Crouch S. P., Slater K. J., Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992 Jul 1;80(1):235–240. [PubMed] [Google Scholar]
- Day C. L., Stowell K. M., Baker E. N., Tweedie J. W. Studies of the N-terminal half of human lactoferrin produced from the cloned cDNA demonstrate that interlobe interactions modulate iron release. J Biol Chem. 1992 Jul 15;267(20):13857–13862. [PubMed] [Google Scholar]
- Derisbourg P., Wieruszeski J. M., Montreuil J., Spik G. Primary structure of glycans isolated from human leucocyte lactotransferrin. Absence of fucose residues questions the proposed mechanism of hyposideraemia. Biochem J. 1990 Aug 1;269(3):821–825. doi: 10.1042/bj2690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991 Oct;88(4):1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hu W. L., Mazurier J., Montreuil J., Spik G. Isolation and partial characterization of a lactotransferrin receptor from mouse intestinal brush border. Biochemistry. 1990 Jan 16;29(2):535–541. doi: 10.1021/bi00454a030. [DOI] [PubMed] [Google Scholar]
- Hurley W. L., Grieve R. C., Magura C. E., Hegarty H. M., Zou S. Electrophoretic comparisons of lactoferrin from bovine mammary secretions, milk neutrophils, and human milk. J Dairy Sci. 1993 Feb;76(2):377–387. doi: 10.3168/jds.S0022-0302(93)77356-7. [DOI] [PubMed] [Google Scholar]
- Hutchens T. W., Henry J. F., Yip T. T. Structurally intact (78-kDa) forms of maternal lactoferrin purified from urine of preterm infants fed human milk: identification of a trypsin-like proteolytic cleavage event in vivo that does not result in fragment dissociation. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2994–2998. doi: 10.1073/pnas.88.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of native and defucosylated lactoferrin. Biochem J. 1983 May 15;212(2):249–257. doi: 10.1042/bj2120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami H., Lönnerdal B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am J Physiol. 1991 Nov;261(5 Pt 1):G841–G846. doi: 10.1152/ajpgi.1991.261.5.G841. [DOI] [PubMed] [Google Scholar]
- Kijlstra A., Kuizenga A., van der Velde M., van Haeringen N. J. Gel electrophoresis of human tears reveals various forms of tear lactoferrin. Curr Eye Res. 1989 Jun;8(6):581–588. doi: 10.3109/02713688908995757. [DOI] [PubMed] [Google Scholar]
- Koczan D., Hobom G., Seyfert H. M. Genomic organization of the bovine alpha-S1 casein gene. Nucleic Acids Res. 1991 Oct 25;19(20):5591–5596. doi: 10.1093/nar/19.20.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclercq Y., Sawatzki G., Wieruszeski J. M., Montreuil J., Spik G. Primary structure of the glycans from mouse serum and milk transferrins. Biochem J. 1987 Nov 1;247(3):571–578. doi: 10.1042/bj2470571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. C., Schryvers A. B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988 Nov;2(6):827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Legrand D., Mazurier J., Colavizza D., Montreuil J., Spik G. Properties of the iron-binding site of the N-terminal lobe of human and bovine lactotransferrins. Importance of the glycan moiety and of the non-covalent interactions between the N- and C-terminal lobes in the stability of the iron-binding site. Biochem J. 1990 Mar 1;266(2):575–581. [PMC free article] [PubMed] [Google Scholar]
- Machnicki M., Zimecki M., Zagulski T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int J Exp Pathol. 1993 Oct;74(5):433–439. [PMC free article] [PubMed] [Google Scholar]
- Makino Y., Nishimura S. High-performance liquid chromatographic separation of human apolactoferrin and monoferric and diferric lactoferrins. J Chromatogr. 1992 Sep 2;579(2):346–349. doi: 10.1016/0378-4347(92)80402-c. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Romm E., Migliorini M. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J Biol Chem. 1994 Sep 23;269(38):23661–23667. [PubMed] [Google Scholar]
- Mazurier J., Legrand D., Hu W. L., Montreuil J., Spik G. Expression of human lactotransferrin receptors in phytohemagglutinin-stimulated human peripheral blood lymphocytes. Isolation of the receptors by antiligand-affinity chromatography. Eur J Biochem. 1989 Feb 1;179(2):481–487. doi: 10.1111/j.1432-1033.1989.tb14578.x. [DOI] [PubMed] [Google Scholar]
- Nichols B. L., McKee K. S., Henry J. F., Putman M. Human lactoferrin stimulates thymidine incorporation into DNA of rat crypt cells. Pediatr Res. 1987 Jun;21(6):563–567. doi: 10.1203/00006450-198706000-00011. [DOI] [PubMed] [Google Scholar]
- Nuijens J. H., Abbink J. J., Wachtfogel Y. T., Colman R. W., Eerenberg A. J., Dors D., Kamp A. J., Strack van Schijndel R. J., Thijs L. G., Hack C. E. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992 Feb;119(2):159–168. [PubMed] [Google Scholar]
- Petschow B. W., Talbott R. D. Response of bifidobacterium species to growth promoters in human and cow milk. Pediatr Res. 1991 Feb;29(2):208–213. doi: 10.1203/00006450-199102000-00021. [DOI] [PubMed] [Google Scholar]
- Platenburg G. J., Kootwijk E. P., Kooiman P. M., Woloshuk S. L., Nuijens J. H., Krimpenfort P. J., Pieper F. R., de Boer H. A., Strijker R. Expression of human lactoferrin in milk of transgenic mice. Transgenic Res. 1994 Mar;3(2):99–108. doi: 10.1007/BF01974087. [DOI] [PubMed] [Google Scholar]
- Reiter B., Brock J. H., Steel E. D. Inhibition of Escherichia coli by bovine colostrum and post-colostral milk. II. The bacteriostatic effect of lactoferrin on a serum susceptible and serum resistant strain of E. coli. Immunology. 1975 Jan;28(1):83–95. [PMC free article] [PubMed] [Google Scholar]
- Rey M. W., Woloshuk S. L., deBoer H. A., Pieper F. R. Complete nucleotide sequence of human mammary gland lactoferrin. Nucleic Acids Res. 1990 Sep 11;18(17):5288–5288. doi: 10.1093/nar/18.17.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik G., Strecker G., Fournet B., Bouquelet S., Montreuil J., Dorland L., van Halbeek H., Vliegenthart J. F. Primary structure of the glycans from human lactotransferrin. Eur J Biochem. 1982 Jan;121(2):413–419. doi: 10.1111/j.1432-1033.1982.tb05803.x. [DOI] [PubMed] [Google Scholar]
- Stowell K. M., Rado T. A., Funk W. D., Tweedie J. W. Expression of cloned human lactoferrin in baby-hamster kidney cells. Biochem J. 1991 Jun 1;276(Pt 2):349–355. doi: 10.1042/bj2760349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez L., Calvo M., Brock J. H. Biological role of lactoferrin. Arch Dis Child. 1992 May;67(5):657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. P., Lo J. Y., Duke M., May G. S., Headon D. R., Conneely O. M. Production of biologically active recombinant human lactoferrin in Aspergillus oryzae. Biotechnology (N Y) 1992 Jul;10(7):784–789. doi: 10.1038/nbt0792-784. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Tomita M., Giehl T. J., Ellison R. T., 3rd Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect Immun. 1993 Feb;61(2):719–728. doi: 10.1128/iai.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziere G. J., van Dijk M. C., Bijsterbosch M. K., van Berkel T. J. Lactoferrin uptake by the rat liver. Characterization of the recognition site and effect of selective modification of arginine residues. J Biol Chem. 1992 Jun 5;267(16):11229–11235. [PubMed] [Google Scholar]
- van der Schoot C. E., von dem Borne A. E., Tetteroo P. A. Characterization of myeloid leukemia by monoclonal antibodies, with an emphasis on antibodies against myeloperoxidase. Acta Haematol. 1987;78 (Suppl 1):32–40. doi: 10.1159/000205900. [DOI] [PubMed] [Google Scholar]