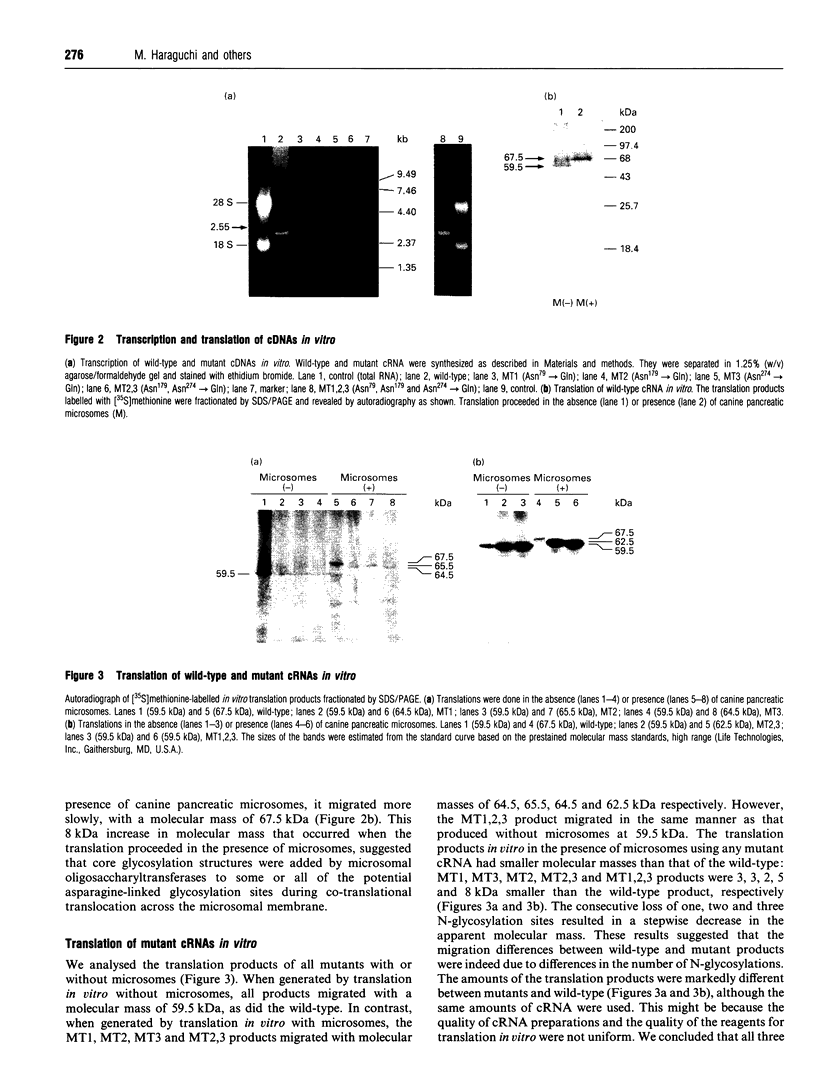
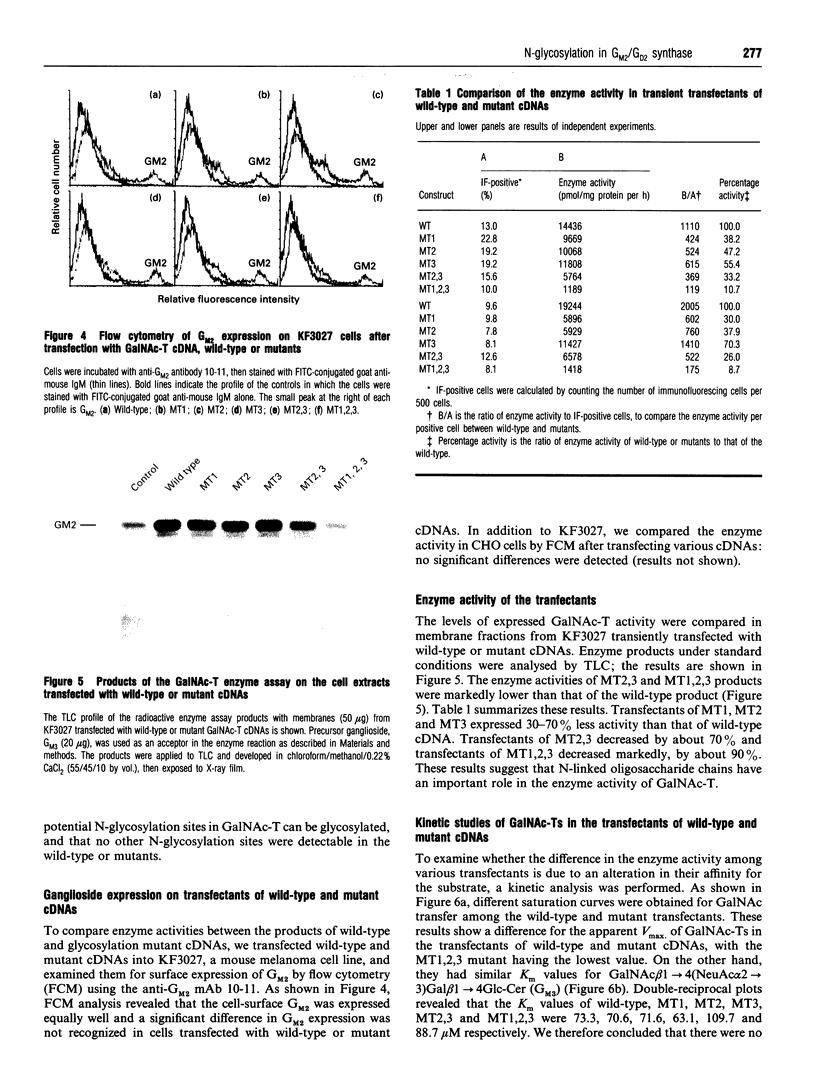
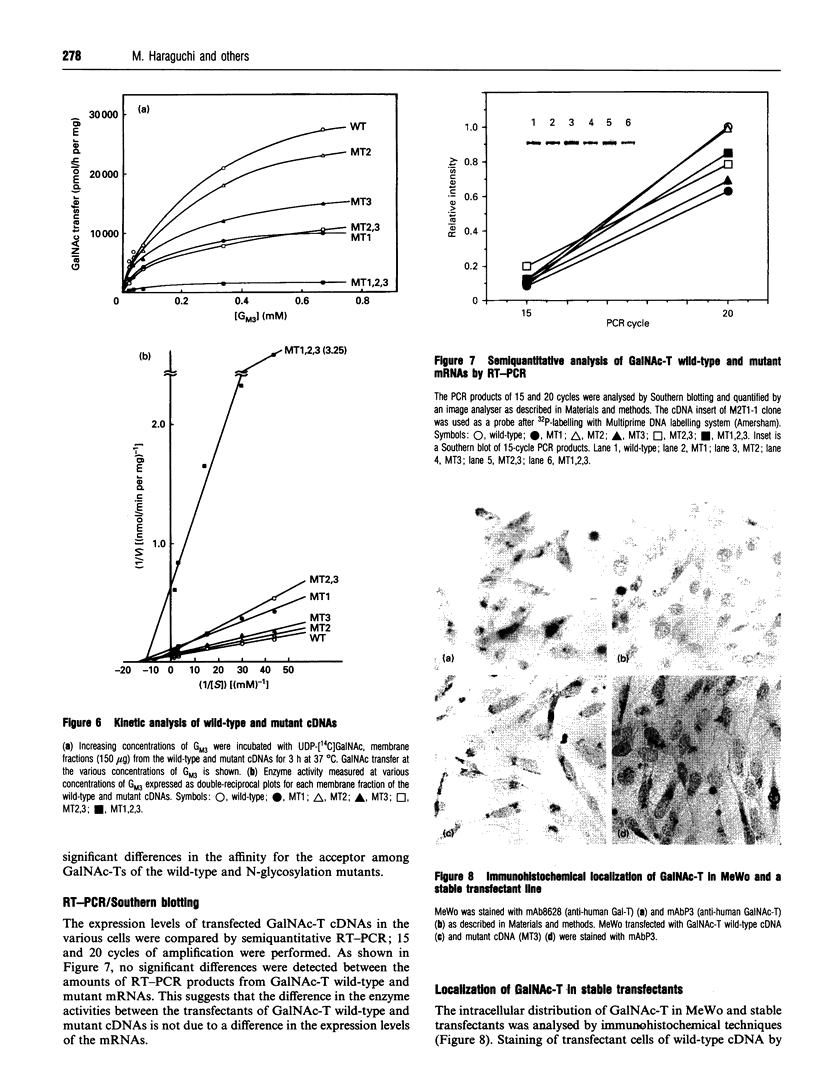
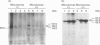Abstract
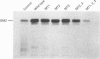
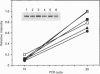
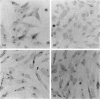
The amino acid sequence deduced from the cloned human cDNA of beta-1,4-N-acetylgalactosaminyltransferase (GalNAc-T; EC 2.4.1.92) gene predicted three potential sites for N-linked glycosylation. Although many glycosyltransferases isolated contain from 2 to 6 N-glycosylation sites, their significance has not been adequately demonstrated. To clarify the roles of N-glycosylation in GalNAc-T function, we generated a series of mutant cDNAs, in which some or all of the glycosylation recognition sites were eliminated by polymerase chain reaction (PCR)-mediated site-directed mutagenesis. Using transcription/translation in vitro, we confirmed that all potential N-glycosylation sites could be used. Although cell lines transfected with mutant cDNAs showed equivalent levels of GalNAc beta 1-->4(NeuAc alpha 2-->3)Gal beta 1-->4Glc-Cer (GM2) to that of the wild-type, the extracts from mutant cDNA transfectants demonstrated lower enzyme activity than in the wild-type. The decrease in enzyme activity was more evident as the number of deglycosylated sites increased, with about 90% decrease in a totally deglycosylated mutant. The enzyme kinetics analysis revealed no significant change of Km among wild-type and mutant cDNA products. The intracellular localization of GalNAc-T expressed in transfectants with wild-type or mutant cDNAs also showed a similar perinuclear pattern (Golgi pattern). These results suggest that N-linked carbohydrates on GalNAc-T are required for regulating the stability of the enzyme structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fast D. G., Jamieson J. C., McCaffrey G. The role of the carbohydrate chains of Gal beta-1,4-GlcNAc alpha 2,6-sialyltransferase for enzyme activity. Biochim Biophys Acta. 1993 Oct 6;1202(2):325–330. doi: 10.1016/0167-4838(93)90023-k. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Furukawa K., Shiku H. Alternatively spliced mRNA of the pX region of human T lymphotropic virus type I proviral genome. FEBS Lett. 1991 Dec 16;295(1-3):141–145. doi: 10.1016/0014-5793(91)81404-v. [DOI] [PubMed] [Google Scholar]
- Gieselmann V., Schmidt B., von Figura K. In vitro mutagenesis of potential N-glycosylation sites of arylsulfatase A. Effects on glycosylation, phosphorylation, and intracellular sorting. J Biol Chem. 1992 Jul 5;267(19):13262–13266. [PubMed] [Google Scholar]
- Higuchi M., Oh-eda M., Kuboniwa H., Tomonoh K., Shimonaka Y., Ochi N. Role of sugar chains in the expression of the biological activity of human erythropoietin. J Biol Chem. 1992 Apr 15;267(11):7703–7709. [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Joziasse D. H. Mammalian glycosyltransferases: genomic organization and protein structure. Glycobiology. 1992 Aug;2(4):271–277. doi: 10.1093/glycob/2.4.271. [DOI] [PubMed] [Google Scholar]
- Koyama T., Hughes R. C. Functional integrins from normal and glycosylation-deficient baby hamster kidney cells. Terminal processing of asparagine-linked oligosaccharides is not correlated with fibronectin-binding activity. J Biol Chem. 1992 Dec 25;267(36):25939–25944. [PubMed] [Google Scholar]
- Kretz K. A., Carson G. S., Morimoto S., Kishimoto Y., Fluharty A. L., O'Brien J. S. Characterization of a mutation in a family with saposin B deficiency: a glycosylation site defect. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2541–2544. doi: 10.1073/pnas.87.7.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukowska-Latallo J. F., Larsen R. D., Nair R. P., Lowe J. B. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. 1990 Aug;4(8):1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- Kumar R., Yang J., Larsen R. D., Stanley P. Cloning and expression of N-acetylglucosaminyltransferase I, the medial Golgi transferase that initiates complex N-linked carbohydrate formation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9948–9952. doi: 10.1073/pnas.87.24.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Boime I. The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J Cell Biol. 1988 Apr;106(4):1049–1059. doi: 10.1083/jcb.106.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag B., Passmore D., Kendrick T., Bhayani H., Sharma S. D. N-linked oligosaccharides of murine major histocompatibility complex class II molecule. Role in antigenic peptide binding, T cell recognition, and clonal nonresponsiveness. J Biol Chem. 1992 Nov 5;267(31):22624–22629. [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1992 Jun 15;267(17):12082–12089. [PubMed] [Google Scholar]
- Natoli E. J., Jr, Livingston P. O., Pukel C. S., Lloyd K. O., Wiegandt H., Szalay J., Oettgen H. F., Old L. J. A murine monoclonal antibody detecting N-acetyl- and N-glycolyl-GM2: characterization of cell surface reactivity. Cancer Res. 1986 Aug;46(8):4116–4120. [PubMed] [Google Scholar]
- Neefjes J. J., De Bruijn M. L., Boog C. J., Nieland J. D., Boes J., Melief C. J., Ploegh H. L. N-linked glycan modification on antigen-presenting cells restores an allospecific cytotoxic T cell response. J Exp Med. 1990 Feb 1;171(2):583–588. doi: 10.1084/jem.171.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O K., Hill J. S., Wang X., McLeod R., Pritchard P. H. Lecithin:cholesterol acyltransferase: role of N-linked glycosylation in enzyme function. Biochem J. 1993 Sep 15;294(Pt 3):879–884. doi: 10.1042/bj2940879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pownall S., Kozak C. A., Schappert K., Sarkar M., Hull E., Schachter H., Marth J. D. Molecular cloning and characterization of the mouse UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I gene. Genomics. 1992 Apr;12(4):699–704. doi: 10.1016/0888-7543(92)90297-6. [DOI] [PubMed] [Google Scholar]
- Qu S. J., Fan H. Z., Blanco-Vaca F., Pownall H. J. Effects of site-directed mutagenesis on the N-glycosylation sites of human lecithin:cholesterol acyltransferase. Biochemistry. 1993 Aug 31;32(34):8732–8736. doi: 10.1021/bi00085a002. [DOI] [PubMed] [Google Scholar]
- Recny M. A., Luther M. A., Knoppers M. H., Neidhardt E. A., Khandekar S. S., Concino M. F., Schimke P. A., Francis M. A., Moebius U., Reinhold B. B. N-glycosylation is required for human CD2 immunoadhesion functions. J Biol Chem. 1992 Nov 5;267(31):22428–22434. [PubMed] [Google Scholar]
- Ruan S., Lloyd K. O. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells: relative glycosyltransferase levels determine ganglioside patterns. Cancer Res. 1992 Oct 15;52(20):5725–5731. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Sarkar M., Hull E., Nishikawa Y., Simpson R. J., Moritz R. L., Dunn R., Schachter H. Molecular cloning and expression of cDNA encoding the enzyme that controls conversion of high-mannose to hybrid and complex N-glycans: UDP-N-acetylglucosamine: alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):234–238. doi: 10.1073/pnas.88.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel A. H., Kemp S., Dollé M., Rudenko G., Wagenaar E. N-glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J Biol Chem. 1993 Apr 5;268(10):7474–7481. [PubMed] [Google Scholar]
- Thampoe I. J., Furukawa K., Vellvé E., Lloyd K. O. Sialyltransferase levels and ganglioside expression in melanoma and other cultured human cancer cells. Cancer Res. 1989 Nov 15;49(22):6258–6264. [PubMed] [Google Scholar]
- Tifft C. J., Proia R. L., Camerini-Otero R. D. The folding and cell surface expression of CD4 requires glycosylation. J Biol Chem. 1992 Feb 15;267(5):3268–3273. [PubMed] [Google Scholar]
- Velan B., Kronman C., Ordentlich A., Flashner Y., Leitner M., Cohen S., Shafferman A. N-glycosylation of human acetylcholinesterase: effects on activity, stability and biosynthesis. Biochem J. 1993 Dec 15;296(Pt 3):649–656. doi: 10.1042/bj2960649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Wright A., Tao M. H., Kabat E. A., Morrison S. L. Antibody variable region glycosylation: position effects on antigen binding and carbohydrate structure. EMBO J. 1991 Oct;10(10):2717–2723. doi: 10.1002/j.1460-2075.1991.tb07819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Akai K., Kawanishi G., Ueda M., Masuda S., Sasaki R. Effects of site-directed removal of N-glycosylation sites in human erythropoietin on its production and biological properties. J Biol Chem. 1991 Oct 25;266(30):20434–20439. [PubMed] [Google Scholar]
- Yamashiro S., Ruan S., Furukawa K., Tai T., Lloyd K. O., Shiku H., Furukawa K. Genetic and enzymatic basis for the differential expression of GM2 and GD2 gangliosides in human cancer cell lines. Cancer Res. 1993 Nov 15;53(22):5395–5400. [PubMed] [Google Scholar]