Abstract
The widely treated order Capnodiales is one of the most important orders in the class Dothideomycetes. Recently, the order Capnodiales s. lat. was reassessed and split into seven orders (Capnodiales s. str., Cladosporiales, Comminutisporales, Mycosphaerellales, Neophaeothecales, Phaeothecales and Racodiales) based on multi-locus phylogeny, morphology and life strategies. In this study, two Arthrocatena strains isolated from sooty mould communities on the leaves of Tiliacordata and needles of Pinusnigra in southern Poland were analyzed. Multi-locus phylogenetic analyses (ITS-LSU-SSU-rpb2-tef1) along with morphological examination showed that they belong to Capnobotryellaantalyensis, which represents a sister taxon to Arthrocatenatenebrosa. Capnobotryellaantalyensis is a rock-inhabiting fungus described from Turkey. The following new combination is proposed: Arthrocatenaantalyensis. Phylogenetic analyses also showed that Arthrocatena and related genus Hyphoconis, both known previously only from rocks, form a sister lineage to orders Cladosporiales and Comminutisporales. The new order Arthrocatenales and new family Arthrocatenaceae are proposed to this clade. Representatives of this order are extremophilic fungi that live on rocks and in sooty mould communities.
Key words: Dothideomycetes, molecular phylogeny, new combination, new family, new order, taxonomy
Introduction
The order Capnodiales in the wide sense (s. lat.) is one of the most important orders in the class Dothideomycetes. It contains thousands of species growing in all areas of the world, the majority of known environments, including most extreme ones, and showing diverse nutritional modes and life strategies (Schoch et al. 2006; Muggia et al. 2008; Crous et al. 2009b, 2013a; Bensch et al. 2012; Groenewald et al. 2013; Hujslová et al. 2013; Quaedvlieg et al. 2013, 2014; Egidi et al. 2014; Wijayawardene et al. 2014; Isola et al. 2016; Duarte et al. 2017; Videira et al. 2017; Gleason et al. 2019; Abdollahzadeh et al. 2020; Czachura et al. 2021; Piątek et al. 2023). This wide concept of the order Capnodiales was recently reassessed by Abdollahzadeh et al. (2020) who split it into seven orders (Capnodiales s. str., Cladosporiales, Comminutisporales, Mycosphaerellales, Neophaeothecales, Phaeothecales and Racodiales) based on multi-locus phylogeny, morphology and life strategies. The redefined order Capnodiales s. str. includes species that are almost exclusively sooty moulds while the remaining orders comprise genera and species generally encompassing other nutritional modes and ecologies (Abdollahzadeh et al. 2020), although Cladosporiales and Mycosphaerellales include some species isolated from sooty mould communities too (e.g., Friend 1965; Flessa et al. 2012, 2021; Flessa and Rambold 2013; Crous et al. 2023a, 2023b; Piątek et al. 2023). The phylogenetic placement of several genera and families within the Capnodiales s. lat. is still unresolved (e.g., Quaedvlieg et al. 2014; Ismail et al. 2016; Abdollahzadeh et al. 2020; Pereira and Phillips 2020). This refers, for example, to two phylogenetically closely related genera Arthrocatena and Hyphoconis that accommodate single species, Arthrocatenatenebrosa and Hyphoconissterilis described as rock-inhabiting fungi from Italian Alps and Mediterranean Spain, respectively (Egidi et al. 2014; Crous et al. 2019a).
Sooty moulds are epiphytes associated with honeydew or sweet plant exudates occurring on the leaves/needles of woody plants (Hughes 1976). Although many species of sooty moulds reside in the order Capnodiales s. str. (Abdollahzadeh et al. 2020) they are also known in other orders and families of the classes Dothideomycetes and Eurotiomycetes (e.g., Friend 1965; Flessa et al. 2012, 2021; Chomnunti et al. 2014; Piątek et al. 2023). Sooty moulds form a multi-species assemblages (Hughes 1976; Hughes and Seifert 2012; Flessa et al. 2012, 2021) containing even 243 species (OTUs) (Dhami et al. 2013) of which many probably remain undescribed.
Fungi isolated from sooty mould communities are sometimes phylogenetically related to rock-inhabiting fungi. Such a relationship was mentioned in the orders Capnodiales (s. lat.) and Chaetothyriales (Chomnunti et al. 2014) and in the genera Lapidomyces (order Mycosphaerellales) and Rachicladosporium (order Cladosporiales) (Crous et al. 2023b; Piątek et al. 2023). Capnobotryellarenispora has been described as a sooty mould associated with other sooty mould Capnobotrysneesii growing on Abiesveitchii branches in Japan (Sugiyama and Amano 1987) and later found on roof tiles that is a habitat resembling rocks (Titze and de Hoog 1990).
In a recent survey of sooty mould communities occurring on ornamental woody plants in urban environments in southern Poland we isolated two strains that were assigned to the genus Arthrocatena based on initial ITS rDNA sequencing. This study aims to identify isolated Arthrocatena strains using morphology and multi-locus phylogenetic analyses and to clarify the phylogenetic placement of the genera Arthrocatena and Hyphoconis within Capnodiales s. lat.
Materials and methods
Isolates
Fungal isolates studied here were obtained from sooty mould communities on ornamental woody plants cultivated in municipal greenery in cities of southern Poland. The initial isolations were made on malt extract agar (MEA – Blakeslee’s formula), potato dextrose agar (PDA), and rose bengal agar (RBC). The details of microbiological media and method of initial isolation are described in Piątek et al. (2023). Dried specimens obtained from cultures are stored in the fungal collection of the W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków (KRAM F). Cultures are deposited in the culture collection of the Westerdijk Fungal Biodiversity Institute (CBS) and in the W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków.
Morphological analyses
Macroscopic characteristics were documented using 4-week-old colonies growing on MEA and PDA incubated at 25 °C. Microscopic characteristics were examined under a Nikon Eclipse 80i light microscope using slide cultures on PDA incubated at 25 °C, after approximately one month growth (Crous et al. 2019b). The disintegration of chains of arthroconidia was observed on MEA cultures. The microscopic structures were measured and photographed using NIS‐Elements BR 3.0 imaging software. Growth at 15 °C and 25 °C on MEA and PDA was assessed by measuring the colony diameter after 2 weeks and 4 weeks.
DNA isolation, amplification and sequencing
DNA was extracted using DNeasy® Plant Mini Kit (Qiagen, Germany), according to the manufacturer’s protocol. Four loci were amplified: ITS1‐5.8S‐ITS2 rDNA (= ITS), fragment of the large subunit rDNA (28S D1–D2 = LSU), the small subunit rDNA (18S = SSU) and protein-coding gene – partial DNA-directed RNA polymerase II second largest subunit (rpb2). The following primer pairs were used for amplification: ITS1–ITS4 for ITS (White et al. 1990), LSU1Fd–LR5 for LSU (Vilgalys and Hester 1990; Crous et al. 2009b), NS1–NS4 for SSU (White et al. 1990), and fRPB2-5F–fRPB2-7cR for rpb2 (Liu et al. 1999). Polymerase chain reactions were performed in a reaction mixture prepared as described in Piątek et al. (2023). ITS and LSU were amplified as described by Czachura et al. (2021). Amplification of SSU was performed with initial denaturation at 94 °C for 3 min followed by 35 cycles of denaturation at 94 °C for 45 sec, the annealing of primers for 30 sec at 52 °C, the elongation at 72 °C for 1 min and the final extension at 72 °C for 10 min. Amplification conditions for rpb2 were set as follows: an initial denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 60 sec, the annealing at 54 °C for 90 sec, the elongation at 72 °C for 2 min and the final extension at 72 °C for 10 min. Amplicons were visualized and verified by gel electrophoresis on 1% agarose gel. Subsequently, the PCR products were enzymatically purified using an Exo-BAP Mix (EURx, Poland) and sequenced bidirectionally by Macrogen Europe B.V. (Amsterdam, the Netherlands). Obtained sequences were assembled and trimmed in Geneious Prime 2020.0.4. Consensus sequences were deposited in the NCBI’s GenBank nucleotide database (https://www.ncbi.nlm.nih.gov/genbank/).
Phylogenetic analyses
The affinity of the isolated strains was first checked in the NCBIs GenBank nucleotide database using the megablast search tool (Zhang et al. 2000). To resolve phylogenetic placement of the isolated strains, the concatenated ITS-LSU-SSU-rpb2-tef1 alignment was assembled. LSU, rpb2 and tef1 sequences of species and strains used for phylogenetic reconstructions were mostly selected from study of Abdollahzadeh et al. (2020). ITS and SSU sequences of the same species and strains were additionally added to the dataset. Finally, the sequences of Arthrocatenatenebrosa, Capnobotryellaantalyensis and Hyphoconissterilis revealed as most closely related to sequences of analyzed strains were also added to the dataset (Table 1).
Table 1.
List of species, with country of origin, host/substrate, strain, GenBank accession numbers and references, used in phylogenetic analyses.
| Species | Country | Host/substrate | Strain | GenBank acc. no. | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | rpb2 | tef1 | |||||
| Aeminiumludgeri | Portugal | limestone | E14 | MG938062 | MG938288 | – | – | – | Trovão et al. 2019 |
| Aeminiumludgeri | Portugal | limestone | E8 | MG938056 | MG938284 | – | – | – | Trovão et al. 2019 |
| Aeminiumludgeri | Portugal | limestone | E12 | MG938054 | MG938286 | – | – | – | Trovão et al. 2019 |
| Amycosphaerellaafricana | South Africa | leaves of Eucalyptusviminalis | CBS 680.95 | MH862549 | KF902048 | – | – | – | Quaedvlieg et al. 2014; Vu et al. 2019 |
| Amycosphaerellakeniensis | Kenya | leaf litter of Eucalyptusgrandis | CBS 111001 | MF951290 | GQ852610 | NG_062384 | MF951433 | – | Crous et al. 2009b, 2009c; Videira et al. 2017 |
| Arthrocatenaantalyensis (syn. Capnobotryellaantalyensis) | Poland | sooty mould community on Tiliacordata | CBS 150720 | OR096278 | OR096282 | OR096280 | OR096699 | – | this study |
| Arthrocatenaantalyensis (syn. Capnobotryellaantalyensis) | Poland | sooty mould community on Pinusnigra | CBS 150721 | OR096279 | OR096283 | OR096281 | OR096700 | – | this study |
| Arthrocatenaantalyensis (syn. Capnobotryellaantalyensis) | Turkey | marble | MA 4659 | AJ972854 | – | AJ972854 | – | – | Sert et al. 2007 |
| Arthrocatenaantalyensis (syn. Capnobotryellaantalyensis) | Turkey | marble | MA 4775 | AJ972860 | – | AJ972860 | – | – | Sert et al. 2007 |
| Arthrocatenatenebrosa | Italy | rock | CCFEE 5413 | NR_144971 | NG_056969 | NG_061095 | – | – | Ruibal et al. 2009; Egidi et al. 2014 |
| Aureobasidiumpullulans | France | Vitisvinifera | AFTOL-ID 912 | – | DQ470956 | DQ471004 | DQ470906 | DQ471075 | Spatafora et al. 2006 |
| Austroafricanaassociata | Australia | Protealepidocarpodendron | CBS 112224 | DQ302968 | KF901827 | GU296200 | – | GU349025 | Crous et al. 2006; Schoch et al. 2009; Quaedvlieg et al. 2014 |
| Batcheloromycessedgefieldii | South Africa | Protearepens | CBS 112119 | NR_137012 | KF937222 | – | – | – | Crous et al. 2008; Quaedvlieg et al. 2014 |
| Capnobotryellarenispora | Japan | Capnobotrysneesii | CBS 214.90 | NR_121295 | NG_058782 | NG_070856 | – | – | Hambleton et al. 2003; Scott et al. 2007; Crous et al. 2009b |
| Capnodiumalfenasii | Brazil | Tabebuia sp. | CBS 146151 | MN749233 | MN749165 | – | MN829260 | MN829346 | Abdollahzadeh et al. 2020 |
| Capnodiumblackwelliae | USA | Myrtuscommunis | CBS 133588 | MN749235 | MH878118 | – | GU371743 | GU349054 | Schoch et al. 2009; Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Capnodiumcoartatum | Thailand | Psidium sp. | MFLUCC 10-0069 | – | JN832614 | JN832599 | – | – | Chomnunti et al. 2011 |
| Capnodiumcoffeae | Zaire | Coffearobusta | CBS 147.52 | MH856967 | GU214400 | DQ247808 | KT216519 | DQ471089 | Spatafora et al. 2006; Crous et al. 2009b; Ismail et al. 2016; Vu et al. 2019 |
| Capnodiumcoffeicola | Thailand | Coffea sp. | MFLUCC 15-0206 | – | KU358920 | – | – | – | Hongsanan et al. 2015b |
| Capnodiumgamsii | Sri Lanka | unknown leaf | CBS 892.73 | MN749237 | GU301847 | – | GU371736 | GU349045 | Schoch et al. 2009; Abdollahzadeh et al. 2020 |
| Capnodiumneocoffeicola | Thailand | Coffeaarabica | CBS 139614 | MN749242 | MN749172 | – | MN829267 | MN829353 | Abdollahzadeh et al. 2020 |
| Capnodiumparacoffeicola | Thailand | Coffeaarabica | CBS 139616 | MN749244 | MN749174 | – | MN829269 | MN829355 | Abdollahzadeh et al. 2020 |
| “Capnodium” salicinum | Indonesia | Bursariaspinosa | CBS 131.34 | MH855469 | EU019269 | DQ677997 | KT216553 | DQ677889 | Schoch et al. 2006b; Crous et al. 2007a; Ismail et al. 2016; Vu et al. 2019 |
| Cercosporabeticola | Italy | Betavulgaris | CBS 116456 | NR_121315 | DQ678091 | NG_062715 | KT216555 | DQ677932 | Groenewald et al. 2005; Schoch et al. 2006b; Ismail et al. 2016 |
| Cercosporellavirgaureae | South Korea | Erigeronannuus | CBS 113304 | GU214658 | KF251805 | GU214658 | KX348051 | – | Crous et al. 2009b; Verkley et al. 2013; Videira et al. 2016 |
| Chaetocapnodiumindonesiacum | Indonesia | Camelliasinensis | CBS 202.30 | MH855113 | GU301849 | GU296178 | MN829273 | GU349060 | Schoch et al. 2009; Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Chaetocapnodiuminsulare | South Africa | Phylicaarborea | CBS 146159 | NR_168830 | MN749178 | – | MN829274 | MN829359 | Abdollahzadeh et al. 2020 |
| Chaetocapnodiumphilippinense | Philippines | palm | MFLUCC 12-0110 | NR_168831 | KP744503 | – | MN829277 | MN829362 | Liu et al. 2015; Abdollahzadeh et al. 2020 |
| Chaetocapnodiumplacitae | Australia | Eucalyptusplacita | CBS 124758 | GQ303268 | GQ303299 | – | MN829278 | MN829363 | Cheewangkoon et al. 2009; Abdollahzadeh et al. 2020 |
| Chaetocapnodiumsiamensis | Thailand | leaves of unidentified plant | MFLUCC 13-0778 | – | KP744479 | – | – | – | Liu et al. 2015 |
| Chaetocapnodiumsummerellii | Australia | Eucalyptusplacita | CBS 146157 | NR_168829 | MN749176 | – | MN829271 | MN829357 | Abdollahzadeh et al. 2020 |
| Chaetocapnodiumtanzanicum | Tanzania | lichen | CBS 145.79 | NR_168832 | MN749182 | – | MN829280 | MN829365 | Abdollahzadeh et al. 2020 |
| Chaetocapnodiumthailandense | Thailand | – | CBS 139619 | NR_168833 | MN749183 | – | MN829281 | MN829366 | Abdollahzadeh et al. 2020 |
| Chaetothyrinaguttulata | Thailand | Mangiferaindica | MFLUCC 15-1080 | KX372277 | KU358917 | KU358916 | – | – | Hongsanan et al. 2017 |
| Chaetothyrinamusarum | Thailand | Musa sp. | MFLUCC 15-0383 | KX372275 | KU710171 | KU710174 | – | – | Singtripop et al. 2016; Hongsanan et al. 2017 |
| Cladosporiumallicinum | Czech Republic | Polygonatumodoratum | CBS 813.71 | – | DQ008149 | – | – | – | Avila et al. 2005 |
| Cladosporiumiridis | Netherlands | Iris sp. | CBS 138.40 | EU167591 | DQ008148 | EU167591 | KT223022 | – | Avila et al. 2005; Simon et al. 2009; Ismail et al. 2016 |
| Cladosporiumramotenellum | United Kingdom | leaves of Arundo sp. | CBS 170.54 | MH857281 | DQ678057 | DQ678004 | DQ677952 | DQ677898 | Schoch et al. 2006b; Vu et al. 2019 |
| Comminutisporaagavacearum | USA | Dasylirionleiophyllum | CBS 619.95 | MH862543 | EU981286 | – | MN829337 | MN829423 | Tsuneda et al. 2008; Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Conidiocarpusasiaticus | Thailand | Coffeaarabica | MFLUCC 10-0062 | KU358924 | JN832612 | JN832597 | – | – | Chomnunti et al. 2011; Hongsanan et al. 2015b |
| Conidiocarpuscaucasicus | Iran | Citrussinensis | GUMH 937 | – | KC833050 | KC833051 | – | – | Bose et al. 2014 |
| Conidiocarpussiamensis | Thailand | Mangiferaindica | MFLUCC 10-0064 | – | JN832609 | JN832594 | – | – | Chomnunti et al. 2011 |
| Cystocoleusebeneus | Austria | – | L161 | – | EU048578 | EU048571 | – | – | Muggia et al. 2008 |
| Cystocoleusebeneus | Austria | – | L348 | – | EU048580 | – | – | – | Muggia et al. 2008 |
| Davidiellomycesaustraliensis | Australia | leaves of Cyperaceae | CPC 29170 | KY979737 | KY979792 | – | LT799790 | – | Crous et al. 2017; Bezerra et al. 2017 |
| Dissoconiumaciculare | Germany | Astragalus sp. | CBS 204.89 | AY725520 | GU214419 | GU214523 | KX288435 | – | Crous et al. 2004, 2009b; Videira et al. 2016 |
| Dissoconiumaciculare | Netherlands | Brassica sp. | CBS 201.89 | AY725519 | GU214418 | GU214522 | KT216557 | – | Crous et al. 2004, 2009b; Ismail et al. 2016 |
| Dissoconiumaciculare | USA | Malusdomestica | CBS 132080 | JQ622083 | JQ622091 | – | – | – | Li et al. 2012 |
| Dissoconiumaciculare | USA | Malusdomestica | CBS 132081 | AY598874 | JQ622097 | – | – | – | Batzer et al. 2005; Li et al. 2012 |
| Dothideainsculpta | France | Clematisvitalba | CBS 189.58 | AF027764 | DQ247802 | DQ247810 | DQ247792 | DQ471081 | Jacobs & Rehner 1998; Schoch et al. 2006a; Spatafora et al. 2006 |
| Dothideasambuci | Austria | Sambucusnigra | AFTOL-ID 274 | DQ491505 | AY544681 | AY544722 | – | – | Lutzoni et al. 2004; James et al. 2006 |
| Dothioracannabinae | India | Daphnecannabina | AFTOL-ID 1359 | NR_144904 | DQ470984 | DQ479933 | DQ470936 | DQ471107 | De Hoog et al. 1999; Spatafora et al. 2006 |
| Dothioraphillyreae | Spain | Phillyreaangustifolia | CBS 473.69 | NR_155057 | EU754146 | EU754047 | – | – | de Gruyter et al. 2009; Crous & Groenewald 2016 |
| Elsinoephaseoli | Cuba | Phaseoluslunatus | AFTOL-ID 1855 | NR_148161 | DQ678095 | DQ678042 | KX887144 | DQ677935 | Schoch et al. 2006b; Fan et al. 2017 |
| Extremusantarcticus | Antarctica | rock | CCFEE 5312 | KF309979 | KF310020 | – | – | – | Egidi et al. 2014 |
| Fumiglobuspieridicola | Canada | Pierisjaponica | UBC F23788 | NR_153985 | KC833052 | NG_065012 | – | – | Bose et al. 2014 |
| Graphiopsischlorocephala | Germany | Paeoniadelavayi | CBS 121522 | EU009457 | EU009457 | – | LT799753 | – | Schubert et al. 2007; Bezerra et al. 2017 |
| Graphiopsischlorocephala | New Zealand | Paeonia sp. | CBS 100405 | EU009456 | EU009456 | – | KT216520 | – | Schubert et al. 2007; Ismail et al. 2016 |
| Heteroconiumcitharexyli | Ecuador | Citharexylumilicifolium | S (type) | HM628776 | HM628775 | – | – | – | Cheewangkoon et al. 2012 |
| Hortaeawerneckii | Greece | sea water-sprayed marble | CBS 100496 | AY128703 | GU301817 | GU296152 | GU371739 | GU349050 | De Leo et al. 2003; Schoch et al. 2009 |
| Houjiayanglingensis | China | Malusdomestica | CBS 125225 | MH863464 | GQ433631 | – | – | – | Yang et al. 2010; Vu et al. 2019 |
| Houjiayanglingensis | China | Malusdomestica | CBS 125226 | GQ433629 | GQ433630 | – | – | – | Yang et al. 2010 |
| Hyalinozasmidiumaerohyalinosporum | Australia | Eucalyptustectifica | CBS 125011 | KF901605 | KF901930 | – | MF951504 | – | Quaedvlieg et al. 2014; Videira et al. 2017 |
| Hyphoconissterilis | Spain | rock | TRN287 | AY843125 | KF310032 | AY843257 | – | _ | Ruibal et al. 2008; Egidi et al. 2014 |
| Leptoxyphiumcacuminum | Thailand | Gossypiumherbaceum | MFLUCC 10-0059 | – | JN832603 | JN832588 | – | – | Chomnunti et al. 2011 |
| Leptoxyphiumcitri | Spain | Citrussinensis | CBS 451.66 | MN749266 | KF902094 | – | GU371727 | GU349039 | Schoch et al. 2009; Quaedvlieg et al. 2014; Abdollahzadeh et al. 2020 |
| Leptoxyphiumglochidion | China | Glochidionwrightii | IFRDCC 2651 | NR_155316 | KF982308 | NG_065036 | – | – | Yang et al. 2014 |
| Leptoxyphiumkurandae | Australia | Eucalyptus sp. | CBS 129530 | JF951150 | JF951170 | – | MN829295 | MN829379 | Crous et al. 2011a; Abdollahzadeh et al. 2020 |
| Leptoxyphiummadagascariense | Madagascar | Eucalyptuscamaldulensis | CBS 124766 | GQ303277 | MH874923 | – | MN829296 | MN829380 | Cheewangkoon et al. 2009; Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Microcyclosporellamali | Slovenia | Malusdomestica | CBS 126136 | MH864045 | GU570547 | – | KX288436 | – | Frank et al. 2010; Videira et al. 2016; Vu et al. 2019 |
| Mycosphaerelloidesmadeirae | Netherlands | Quercusrobur | CBS 116066 | AY853188 | KX286989 | – | KX288444 | – | Videira et al. 2016 |
| Myriangiumhispanicum | – | Acermonspessulanum | CBS 247.33 | MH855426 | GU301854 | GU296180 | GU371744 | GU349055 | Schoch et al. 2009; Vu et al. 2019 |
| Neoantennariellaphylicae | United Kingdom | Phylicaarborea | CBS 146163 | NR_168834 | MN749211 | – | MN829313 | MN829397 | Abdollahzadeh et al. 2020 |
| Neoasbolisiaphylicae | United Kingdom | Phylicaarborea | CBS 146168 | NR_168835 | MN749215 | – | MN829317 | MN829401 | Abdollahzadeh et al. 2020 |
| Neocladosporiumleucadendri | South Africa | Leucadendron sp. | CBS 131317 | NR_152324 | JQ044455 | – | LT799755 | – | Crous et al. 2011b; Bezerra et al. 2017 |
| Neodevriesiahilliana | New Zealand | Macrozamiacommunis | CBS 123187 | NR_145098 | GU214414 | – | LT799761 | – | Crous et al. 2009b; Bezerra et al. 2017 |
| Neodevriesiamodesta | Italy | rock | CBS 137182 | NR_144975 | KF310026 | – | – | – | Egidi et al. 2014 |
| Neodevriesiapakbiae | Thailand | unidentified fern | CBS 139914 | NR_137997 | KR476775 | – | – | – | Crous et al. 2015 |
| Neodevriesiastirlingiae | Australia | Stirlingialatifolia | CBS 133581 | NR_120228 | KC005799 | – | – | – | Crous et al. 2012 |
| Neodevriesiastrelitziae | South Africa | Strelitzianicolai | CBS 122379 | NR_175123 | GU301810 | NG_078729 | GU371738 | GU349049 | Arzanlou et al. 2008; Schoch et al. 2009; Vu et al. 2019 |
| Neodevriesiaxanthorrhoeae | Australia | Xanthorrhoeaaustralis | CBS 128219 | NR_144962 | HQ599606 | – | – | – | Crous et al. 2010 |
| Neomycosphaerellapseudopentameridis | South Africa | Pseudopentamerismacrantha | CBS 136407 | KF777173 | KF777226 | – | MF951545 | – | Crous et al. 2013b; Videira et al. 2017 |
| Neophaeothecasalicorniae | South Africa | Salicornia sp. | CBS 141299 | NR_145401 | KX228327 | – | MN829343 | MN829429 | Crous et al. 2016; Abdollahzadeh et al. 2020 |
| Neophaeothecatriangularis | Belgium | wet surface of humidifier of air conditioning unit | CBS 471.90 | MH862225 | EU019279 | – | MN829344 | MN829430 | Crous et al. 2007a; Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Neoramulariopsiscatenulata | Rwanda | Phaseolusvulgaris | CBS 355.73 | NR_153920 | KX286973 | – | KX288424 | – | Videira et al. 2016 |
| Paradevriesiacompacta | Spain | rock | CBS 118294 | NR_144955 | GU323220 | NG_064945 | GU371751 | GU349088 | Ruibal et al. 2005, 2009; Schoch et al. 2009 |
| Paramycosphaerellaintermedia | New Zealand | Eucalyptussaligna | CBS 114356 | NR_164413 | KF902026 | – | – | – | Quaedvlieg et al. 2014; Lee et al. 2016 |
| Paramycosphaerellamarksii | South Africa | Eucalyptusgrandis | CBS 110750 | DQ267596 | DQ204757 | – | – | – | Hunter et al. 2006 |
| Penidiella sp. | – | – | CPC 16707 | MN749304 | MN749230 | – | MN829339 | MN829425 | Abdollahzadeh et al. 2020 |
| Petrophilaincerta | Spain | rock | CBS 118608 | NR_144956 | KF310030 | – | – | – | Ruibal et al. 2005; Egidi et al. 2014 |
| Phaeothecafissurella | Canada | Pinuscontorta | CBS 520.89 | MH862184 | GU117900 | NG_065804 | MN829342 | MN829428 | Sterflinger et al. 1999; Yang et al. 2010; Vu et al. 2019 |
| Phaeothecoidiellaillinoisensis | USA | Malus sp. | CBS 125223 | NR_137740 | GU117901 | – | – | – | Yang et al. 2010 |
| Phaeothecoidiellamissouriensis | USA | Malus sp. | CBS 118959 | GU117899 | GU117903 | – | – | – | Yang et al. 2010 |
| Phaeoxyphiellaaustraliana | Australia | Agonis sp. | CBS 146169 | NR_168837 | MN749220 | – | MN829322 | MN829406 | Abdollahzadeh et al. 2020 |
| Phaeoxyphiellaphylicae | United Kingdom | Phylicaarborea | CBS 146170 | NR_168836 | MN749219 | – | MN829321 | MN829405 | Abdollahzadeh et al. 2020 |
| Phloeosporaulmi | Austria | Ulmusglabra | CBS 344.97 | KF251202 | KF251705 | – | – | – | Quaedvlieg et al. 2013 |
| Phragmocapniasbetle | Thailand | Ixora sp. | MFLUCC 10-0053 | KU358922 | JN832606 | JN832591 | – | – | Chomnunti et al. 2011; Hongsanan et al. 2015b |
| Phragmocapniasplumeriae | Thailand | Plumeria sp. | MFLUCC 15-0205 | KU358919 | KU358918 | – | – | – | Hongsanan et al. 2015b |
| Polychaetoncitri | Iran | Citrusaurantium | CBS 116435 | GU214649 | GU214469 | – | MN829310 | MN829394 | Crous et al. 2009b; Abdollahzadeh et al. 2020 |
| Pseudoveronaeaellipsoidea | USA | Malusdomestica | CBS 132085 | NR_111367 | FJ147154 | – | KT921165 | – | Diaz Arias et al. 2010; Ismail et al. 2016 |
| Pseudoveronaeaobclavata | USA | Malusdomestica | CBS 132086 | NR_111168 | JQ622102 | – | – | – | Batzer et al. 2005; Li et al. 2012 |
| Pseudozasmidiumeucalypti | Australia | Eucalyptustereticornis | CBS 121101 | KF901606 | KF901931 | – | MF951637 | – | Quaedvlieg et al. 2014; Videira et al. 2017 |
| Rachicladosporiumamericanum | USA | leaf litter | CBS 124774 | NR_175021 | GQ303323 | – | MN829336 | MN829421 | Cheewangkoon et al. 2009; Abdollahzadeh et al. 2020 |
| Rachicladosporiumcboliae | USA | twig | CBS 125424 | MH863703 | GU214484 | NG_062827 | LT799763 | MN829422 | Crous et al. 2009b; Bezerra et al. 2017; Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Rachicladosporiumeucalypti | Ethiopia | Eucalyptusglobulus | CBS 138900 | NR_155718 | KP004476 | – | – | – | Crous et al. 2014 |
| Rachicladosporiumpini | Netherlands | Pinusmonophylla | CBS 129525 | JF951145 | JF951165 | – | LT799764 | – | Crous et al. 2011a; Bezerra et al. 2017 |
| Racodiumrupestre | Austria | – | L346 | GU067666 | EU048583 | EU048575 | – | – | Muggia et al. 2008; Muggia & Grube 2010 |
| Racodiumrupestre | United Kingdom | – | L423 | GU067668 | EU048581 | – | – | – | Muggia et al. 2008; Muggia & Grube 2010 |
| Racodiumrupestre | Italy | – | L424 | GU067669 | EU048582 | – | – | – | Muggia et al. 2008; Muggia & Grube 2010 |
| Ramichloridiumluteum | China | Malusdomestica | CBS 132088 | NR_119684 | JQ622099 | – | MF951417 | – | Li et al. 2012; Videira et al. 2017 |
| Ramulariaendophylla | Netherlands | Quercusrobur | CBS 113265 | KF251220 | KF251723 | – | – | – | Quaedvlieg et al. 2013 |
| Ramularianyssicola | USA | Nyssaogeche x sylvatica | CBS 127665 | NR_111549 | NG_070531 | – | KJ504636 | – | Minnis et al. 2011; Videira et al. 2015a |
| Ramulariapusilla | Germany | Poaannua | CBS 124973 | NR_154917 | KP894141 | – | KP894687 | – | Videira et al. 2015b |
| Readeriellanontingens | Australia | Eucalyptusoblonga | CPC 14444 | KF901726 | KF902073 | – | – | – | Quaedvlieg et al. 2014 |
| Readerielliopsisfuscoporiae | French Guiana | Fuscoporiawahlbergii | CBS 139900 | NR_137978 | KR476755 | – | MN829326 | MN829410 | Crous et al. 2015; Abdollahzadeh et al. 2020 |
| Readerielliopsisguyanensis | French Guiana | decaying leaf | CBS 117550 | NR_176103 | FJ493211 | – | MN829327 | MN829411 | Crous et al. 2008; Abdollahzadeh et al. 2020 |
| Saxophilatyrrhenica | Italy | stone monument | CCFEE 5935 | KP791764 | NG_059571 | – | – | – | Isola et al. 2016 |
| Schismatommadecolorans | – | – | AFTOL-ID 307 | AY548808 | AY548815 | AY548809 | DQ883715 | DQ883725 | Lutzoni et al. 2004; Spatafora et al. 2006 |
| Schizothyriumcryptogama | USA | Malusdomestica | CBS 125658 | FJ425208 | FJ147157 | – | KT216548 | – | Diaz Arias et al. 2010; Ismail et al. 2016 |
| Schizothyriumpomi | USA | Malusdomestica | CBS 125312 | FJ425206 | FJ147155 | – | KT216539 | – | Diaz Arias et al. 2010; Ismail et al. 2016 |
| Schizothyriumwisconsinensis | USA | Malusdomestica | CBS 125659 | FJ425209 | FJ147158 | – | KT216549 | – | Diaz Arias et al. 2010; Ismail et al. 2016 |
| Scolecoxyphiumblechni | United Kingdom | Blechnumpalmiforme | CBS 146174 | NR_168838 | MN749224 | – | MN829328 | MN829412 | Abdollahzadeh et al. 2020 |
| Scolecoxyphiumblechnicola | United Kingdom | Blechnumpalmiforme | CBS 146175 | NR_168839 | MN749225 | – | MN829329 | MN829413 | Abdollahzadeh et al. 2020 |
| Scolecoxyphiumleucadendri | South Africa | Leucadendron sp. | CBS 146176 | NR_168840 | MN749226 | – | MN829330 | MN829414 | Abdollahzadeh et al. 2020 |
| Scolecoxyphiumphylicae | South Africa | Phylicaarborea | CBS 146177 | NR_168841 | MN749227 | – | MN829331 | MN829415 | Abdollahzadeh et al. 2020 |
| Scoriasaphidis | – | aphid | CBS 325.33 | GU214696 | MH866910 | – | KT216542 | MN829417 | Crous et al. 2009b; Ismail et al. 2016; Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Scoriascamelliae | Indomesia | Camelliasinensis | CBS 201.30 | MH855112 | MH866560 | – | MN829333 | MN829418 | Vu et al. 2019; Abdollahzadeh et al. 2020 |
| Scoriasleucadendri | South africa | Leucadendronmuirii | CBS 131318 | JQ044437 | JQ044456 | – | MN829334 | MN829419 | Crous et al. 2011b; Abdollahzadeh et al. 2020 |
| Scoriasmangiferae | Thailand | Mangiferaindica | MFLUCC 15-0230 | NR_154422 | KT588603 | – | – | – | Hongsanan et al. 2015a |
| Scoriasspongiosa | Thailand | Entada sp. | MFLUCC 10-0084 | – | JN832601 | JN832586 | – | – | Chomnunti et al. 2011 |
| Septorialycopersici | South Korea | Lycopersiconesculentum | CBS 128654 | MH865102 | KF251966 | – | KX348091 | – | Verkley et al. 2013; Videira et al. 2016; Vu et al. 2019 |
| Septoriaprotearum | South Africa | Zantedeschiaaethiopica | CBS 135477 | KF251524 | KF252029 | – | – | – | Verkley et al. 2013 |
| Sporidesmajorapennsylvaniensis | USA | Malusdomestica | CBS 125229 | NR_156639 | MF951122 | – | MF951424 | – | Videira et al. 2017 |
| Stomiopeltisversicolor | USA | Malusdomestica | GA3-23C2b | FJ438375 | FJ147163 | – | – | – | Diaz Arias et al. 2010 |
| Teratosphaeriastellenboschiana | South Africa | Eucalyptuspunctata | CBS 125215 | KF901733 | KF937247 | – | – | – | Quaedvlieg et al. 2014 |
| Teratosphaeriaceae sp. | – | – | CPC 16695 | MN749303 | MN749231 | – | MN829340 | MN829426 | Abdollahzadeh et al. 2020 |
| Teratosphaeriaceae sp. | – | – | CPC 17588 | MN749305 | MN749232 | – | MN829341 | MN829427 | Abdollahzadeh et al. 2020 |
| Uwebrauniacommune | South Africa | Eucalyptusnitens | CBS 110747 | AY725535 | GU214420 | GU214525 | KT216558 | – | Crous et al. 2004, 2009b; Ismail et al. 2016 |
| Verrucocladosporiumdirinae | United Kingdom | Dirinamassiliensis | CBS 112794 | EU040244 | EU040244 | – | – | – | Crous et al. 2007b |
| Xenodevriesiastrelitziicola | South Africa | Strelitzia sp. | CBS 122480 | NR_171741 | NG_059085 | – | – | – | Crous et al. 2009b; Vu et al. 2019 |
| Xenomycosphaerellaelongata | Venezuela | Eucalyptuscamaldulensis x urophylla | CBS 120735 | NR_154469 | JF700942 | – | MF951687 | – | Crous et al. 2007c; Quaedvlieg et al. 2011; Videira et al. 2017 |
| Zasmidiumpseudotsugae | USA | Pseudotsugamenziesii | rapssd | EF114687 | EF114704 | EF114729 | – | – | Winton et al. 2007 |
| Zasmidiumtsugae | USA | Tsugaheterophylla | ratstk | EF114688 | EF114705 | EF114730 | – | – | Winton et al. 2007 |
AFTOL-ID: Assembling the Fungal Tree of Life (AFTOL); CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CCFEE = Culture Collection of Fungi from Extreme Environments, Tuscia University, Viterbo, Italy; CPC: Culture collection of Pedro Crous, housed at Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; GUMH: Guilan University Mycological Herbarium, Rasht, Iran; IFRDCC: International Fungal Research & Development Centre Culture Collection, Chinese Academy of Forestry, Kunming, China; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; S: Herbarium of the Swedish Museum of Natural History, Stockholm, Sweden; UBC: Herbarium of the University of British Columbia, Vancouver, Canada. Unknown abbreviations: E, GA3-23C2b, L, MA, rapssd, ratstk, TRN – indicates unavailable data or sequence.
For phylogenetic analyses, sequences were separately aligned for each single-gene dataset using MAFFT algorithm (Katoh et al. 2005) in Geneious 11.1.5. The phylogenetic reconstructions were performed using the concatenated ITS-LSU-SSU-rpb2-tef1 alignment. Maximum likelihood (ML) analysis was conducted using RAxML-NG v. 1.1.1 (Kozlov et al. 2019), with a bootstrap of 1000 replicates. Bayesian inference (BI) analysis was carried out using MrBayes v. 3.2.6 (Ronquist et al. 2012). For both ML and BI analyses, the ModelTest-NG v. 0.2.0 was used to select the best substitution models using Bayesian Information Criterion (BIC) (Darriba et al. 2020). BI analysis was performed by running 2 000 000 generations in four chains, saving the current tree to file every 100 generations. The first 25% of trees were discarded as burn-in. Average standard deviations of split frequencies were <0.01 at the end of the runs. The final phylogenetic trees were visualized using FigTree v1.4.3. The alignment was deposited at figshare.com (https://doi.org/10.6084/m9.figshare.25623660.v1).
Results
Phylogenetic analyses
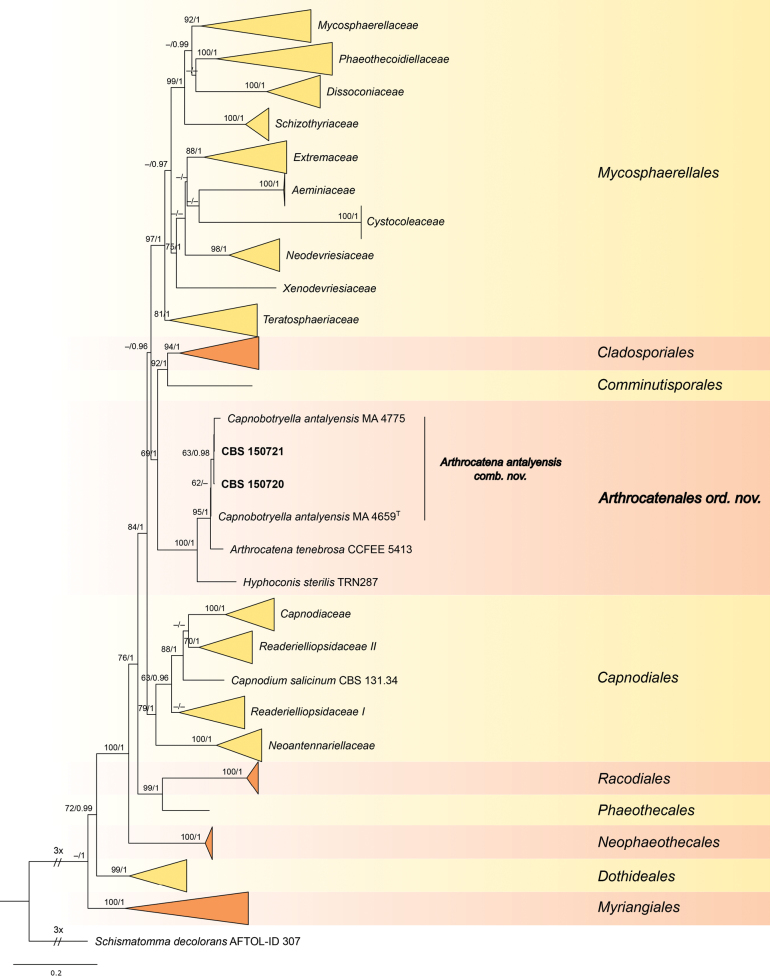
The concatenated ITS-LSU-SSU-rpb2-tef1 alignment contained sequences belonging to 130 species, including Schismatommadecolorans used as an outgroup. The alignment comprised a total of 4829 characters (ITS: 829, LSU: 819, SSU: 946, rpb2: 1122, tef1: 1113), including alignment gaps. The best matching substitution models selected for single locus alignments in the ML analysis were as follows: GTR+I+G4 for ITS, TIM3+I+G4 for LSU, K80+I+G4 for SSU, TIM2+I+G4 and TPM3uf+I+G4 for rpb2 (three codons), and JC+I+G4, HKY+I+G4 and TIM3+I+G4 for tef1 (three codons). The BI analysis was performed with the following substitution models: GTR+I+G4 for ITS and LSU, K80+I+G4 for SSU, GTR+I+G4 and HKY+I+G4 for rpb2 codons, and JC+I+G4, HKY+I+G4 and HKY+G4 for tef1 codons. ML and BI analyses resulted in similar tree topologies (Suppl. materials 1, 2). The best scoring maximum likelihood phylogenetic tree is shown on Fig. 1. Maximum likelihood bootstrap (MLB) support values above 60% and Bayesian posterior probabilities (BPP) above 0.95 are shown at the nodes.
Figure 1.
Reduced phylogenetic tree of selected members of the Capnodiales s. lat., Dothideales and Myriangiales, including all described species of the genera Arthrocatena and Hyphoconis, obtained from a maximum likelihood analysis of the combined multi-locus alignment (ITS, LSU, SSU, rpb2, tef1). The positions of new strains, Arthrocatenaantalyensis comb. nov. and new order Arthrocatenales are indicated in bold. Ex-type cultures are indicated with superscript T. Numbers above branches indicate maximum likelihood bootstrap (MLB) support values > 60% and Bayesian posterior probabilities (BPP) > 0.95, respectively (MLB/BPP). Schismatommadecolorans was used as an outgroup. The scale bar represents the expected number of changes per site.
The sequences of analyzed strains CBS 150720 and CBS 150721 clustered with sequences of type and additional strain of Capnobotryellaantalyensis in a moderately supported clade (only in ML: MLB = 62%), which was sister to Arthrocatenatenebrosa with high support (MLB = 95%, BPP = 1). Capnobotryellaantalyensis is recombined here to Arthrocatena. The sequence similarity between different strains of Arthrocatenaantalyensis ranges between 97.4% and 98.7% in ITS. The sequence similarity between A.antalyensis and A.tenebrosa is 96.1–96.9% in ITS. Members of Arthrocatena formed a fully supported sister clade to single species lineage representing the genus and species Hyphoconissterilis. The clustering of Arthrocatena + Hyphoconis was resolved at sister position (MLB = 69%, BPP = 1) to clades representing orders Cladosporiales and Comminutisporales. The new order Arthrocatenales and new family Arthrocatenaceae are proposed to this clade.
Taxonomy
. Arthrocatenales
Piątek, Stryjak-Bogacka & Czachura ord. nov.
7DC36CED-6119-5ACA-9F07-299205F5FDE5
854789
Etymology.
Named after the genus Arthrocatena.
Description.
Colonies erumpent, spreading, with elevated and folded center, greenish olivaceous, forming concentric rings, margin smooth, entire or undulate. Reverse black. Mycelium composed of branched, septate, pale brown or brown, smooth, straight, flexuose or torulose, thin-walled hyphae. Arthroconidia ellipsoid or broadly ellipsoid, rarely barrel-shaped, brown, smooth, one-septate, intercalary or on side branches, single or in chains. Chlamydospore-like cells spherical, brown, smooth, aseptate, intercalary, in simple or branched chains. Chlamydospores spherical, brown, smooth, muriformly septate, intercalary, single.
Type family.
Arthrocatenaceae Piątek, Stryjak-Bogacka & Czachura.
. Arthrocatenaceae
Piątek, Stryjak-Bogacka & Czachura fam. nov.
3046409E-AFC1-5F9A-A2AF-385807D2E6F3
854790
Etymology.
Named after the genus Arthrocatena.
Description.
Colonies erumpent, spreading, with elevated and folded center, greenish olivaceous, forming concentric rings, margin smooth, entire or undulate. Reverse black. Mycelium composed of branched, septate, pale brown or brown, smooth, straight, flexuose or torulose, thin-walled hyphae. Arthroconidia ellipsoid or broadly ellipsoid, rarely barrel-shaped, brown, smooth, one-septate, intercalary or on side branches, single or in chains. Chlamydospore-like cells spherical, brown, smooth, aseptate, intercalary, in simple or branched chains. Chlamydospores spherical, brown, smooth, muriformly septate, intercalary, single.
Type genus.
Arthrocatena Egidi & Selbmann.
. Arthrocatena antalyensis
(Sert & Sterfl.) Piątek, Stryjak-Bogacka & Czachura comb. nov.
014183E8-D106-5ADF-B217-6DC3228A1762
854791
Figure 2.
Morphology of Arthrocatenaantalyensis (strain CBS 150720, e–h slide culture on PDA): a, b general view and detailed view of upper side of colony on MEA after 4 weeks of growth at 25 °C c, d general view and detailed view of upper side of colony on PDA after 4 weeks of growth at 25 °C e general view of hyphae f, g straight hyphae (note anastomosing hyphae visible on figure g) h terminal, flexuose and torulose hyphae. Scale bars: 50 µm (e); 10 µm (f–h).
Figure 3.
Morphology of Arthrocatenaantalyensis (strain CBS 150720, slide culture on PDA): a general view of hyphae, arthroconidia and chlamydospore b–d arthroconidia e chlamydospore-like cells f–g chlamydospore-like cells and muriformly septate chlamydospores (indicated by arrows). Scale bars: 50 µm (a); 10 µm (b–g).
Figure 4.
Morphology of Arthrocatenaantalyensis (strain CBS 150720, on MEA): a, b arthroconidia, arrows indicate disintegrating chains of arthroconidia c–e disintegrated arthroconidia. Scale bars: 10 µm (a–e).
Basionym.
Capnobotryellaantalyensis Sert & Sterfl., Mycol. Res. 111(10): 1237 (2007).
Typus.
Turkey, Antalya, isolated from the surface of a child’s grave in Side museum (holotype: ACBR MA 4659).
DNA barcodes (from analysed strains).
ITS (OR096278, OR096279), LSU (OR096282, OR096283), SSU (OR096280, OR096281), rpb2 (OR096699, OR096700).
Description.
Mycelium composed of branched, septate, pale brown or brown, smooth, straight, flexuose or torulose, thin-walled hyphae, 3.5–7.0 µm wide, consisting of elongated, subglobose, broadly ellipsoidal or pyriform cells, sometimes anastomosing; hyphae develop into arthroconidia, chlamydospore-like cells or chlamydospores. Arthroconidia ellipsoid or broadly ellipsoid, rarely barrel-shaped, brown, smooth, one-septate, 9.0–19.0(–23.0) × 6.5–8.5 µm, produced intercalary or rarely on side branches, single or in chains. Chlamydospore-like cells spherical, brown, smooth, aseptate, 7.0–12.0 × 7.0–10.0 µm, produced intercalary, in simple or branched chains. Chlamydospores spherical, brown, smooth, muriformly septate, 12.5–15.0 × 11.0–14.0 µm, produced intercalary, single between chlamydospore-like cells.
Culture characteristics.
Colonies on MEA erumpent, spreading, with elevated and folded center, greenish olivaceous, forming concentric rings, reaching 8 mm diam after 4 weeks growth at 15 °C and 12 mm diam after 4 weeks growth at 25 °C, surface with moderate aerial mycelium, margin smooth and entire, darker than the remaining part. Reverse black. Colonies on PDA erumpent, spreading, with elevated and folded center, greenish olivaceous, forming indistinct concentric rings, reaching 10 mm diam after 4 weeks growth at 15 °C and 14 mm diam after 4 weeks growth at 25 °C, surface with sparse aerial mycelium, margin smooth and undulate, concolours with the remaining part. Reverse black.
Specimens examined.
Poland, Silesian Province, Katowice County: Katowice-Bogucice, municipal greenery, isolated from sooty mould community on Tiliacordata leaves, 10 Sept. 2018, leg. M. Piątek, W. Bartoszek & P. Czachura (KRAM F-59837; culture: G57 = CBS 150720); Podkarpackie Province, Rzeszów County: Rzeszów–Generała Władysława Andersa, municipal greenery, isolated from sooty mould community on Pinusnigra needles, 17 Sept. 2018, leg. M. Piątek, W. Bartoszek & P. Czachura (KRAM F-59838; culture: G385 = CBS 150721).
Notes.
Arthrocatenaantalyensis differs from Arthrocatenatenebrosa in having larger arthroconidia (6.0–11.5 × 3.0–5.5 μm in A.tenebrosa; Egidi et al. 2014) and formation of chlamydospore-like cells and muriformly septate chlamydospores.
Discussion
Sooty moulds and communities formed by these fungi are still understudied and for that reason they are probably a rich source of interesting or undescribed species. Here, two Arthrocatena strains isolated from sooty mould communities on leaves of Tiliacordata and needles of Pinusnigra in southern Poland were analyzed. Interestingly, in the multi-locus phylogenetic analyses the sequences of the sooty mould strains grouped together with sequences of type and additional strain of Capnobotryellaantalyensis (MA 4659 and MA 4775), a rock-inhabiting fungus described from two sites in Turkey (Sert et al. 2007). Support for branch uniting four strains of C.antalyensis is moderate and genetic differences between them are relatively high but they are assigned to the same species due to similarities in their micromorphological features. Apart from morphological differences Capnobotryellaantalyensis is well separated phylogenetically from its sister species Arthrocatenatenebrosa. The affinity of C.antalyensis to A.tenebrosa was previously shown by Laichmanová (2023) on the phylogenetic tree resolving position of some rock-inhabiting fungal strains from Antarctica. On the other hand, on the phylogenetic trees published by Zucconi et al. (2012), Isola et al. (2016) and De Leo et al. (2022) three strains assigned to Capnobotryellaantalyensis (MA 4615, MA 4624, MA 4766) formed a distinct lineage within the current genus Neodevriesia. However, none of these strains were originally cited in the protologue of C.antalyensis (Sert et al. 2007) and represent other rock-inhabiting fungus, probably undescribed species of Neodevriesia. The type species of the genus Capnobotryella, C.renispora, is a member of the family Teratosphaeriaceae in the Mycosphaerellales (Crous et al. 2009b; Delgado et al. 2018; Li et al. 2020; this study). Therefore, Capnobotryellaantalyensis is reallocated to the genus Arthrocatena.
The phylogenetic placement of Arthrocatena and its sister genus Hyphoconis remained unclear. In a study of Egidi et al. (2014), where these two genera were described, they formed a distinct clade within the order Capnodiales s. lat. that was positioned either as sister to clade now assigned to the order Mycosphaerellales or as sister to clades representing current orders Cladosporiales and Mycosphaerellales. In a study of Hongsanan et al. (2020) the genus Hyphoconis was placed as sister to clade now assigned to the order Cladosporiales. Consequently, Arthrocatena and Hyphoconis were included in Capnodiales incertae sedis when using wide concept of the order (Wijayawardene et al. 2014, 2017; Hongsanan et al. 2020) or Mycosphaerellales incertae sedis when using current concept of the capnodialean orders (Wijayawardene et al. 2022).
Our molecular phylogenetic analyses of the concatenated ITS-LSU-SSU-rpb2-tef1 alignment showed that Arthrocatena and Hyphoconis form a distinct lineage sister to orders Cladosporiales and Comminutisporales. Therefore, a new order Arthrocatenales is described to accommodate these two genera. These three orders have some ecological and morphological peculiarities that differentiate them. Cladosporiales accommodates hundreds of species that are mostly saprobic, rarely lichenicolous, endolithic, endophytic or plant parasitic and distributed over the whole world. They usually produce solitary conidiophores with chains of pigmented conidia, which germinate and grow very quickly on culture media (Abdollahzadeh et al. 2020). Sexual morph is rarely observed in Cladosporiales but, if present, is mycosphaerella-like with pseudothecial ascomata and one-septate ascospores (Bensch et al. 2012; Crous et al. 2014, 2017). Comminutisporales includes only one species, Comminutisporaagavacearum (with its asexual morph known as Hyphosporaagavacearum), which inhabits dead leaves of Dasylirionleiophyllum and Nolina sp. (Asparagaceae) in Texas and New Mexico, USA. In the sexual stage it produces pseudothecial, uniloculate ascomata and muriformly septate ascospores, while in the asexual stage it forms hyphae with cellular clumps containing numerous endoconidia (Ramaley 1996; Zalar et al. 1999; Abdollahzadeh et al. 2020). Newly described order Arthrocatenales includes only two genera and three species that are extremophilic fungi isolated from rocks or sooty mould communities. All described species in the Arthrocatenales are known only from sterile mycelia (Hyphoconis) that also produce arthroconidia and chlamydospores (Arthrocatena).
Arthrocatena has been reported, in different and mostly metabarcoding studies, from gut of feather mites in Spain (Doña et al. 2019), plants in China, Estonia and Italy (Wu et al. 2019; Giampetruzzi et al. 2020; Küngas et al 2020), indoor dust in the USA (Cox et al. 2022), tsetse fly in Tanzania (Kim et al. 2022) or rocks in Antarctica (Laichmanová 2023). This suggests that the ecological spectrum and distribution of Arthrocatenales may be wider than currently known. However, cultures and multi-locus phylogenetic analyses are necessary to resolve species assignments of fungi detected in the metabarcoding studies.
Supplementary Material
Acknowledgements
We are grateful to Wacław Bartoszek (Kraków, Poland) for help in the field work.
Citation
Piątek M, Stryjak-Bogacka M, Czachura P (2024) Arthrocatenales, a new order of extremophilic fungi in the Dothideomycetes. MycoKeys 108: 47–74. https://doi.org/10.3897/mycokeys.108.128033
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
This study was funded by the National Science Centre, Poland, under the project 2017/27/B/NZ9/02902.
Author contributions
MP: conceptualization, investigation, formal analyses, visualisation, writing – original draft preparation; MSB: investigation, formal analyses, visualisation, writing – review and editing; PC: investigation, writing – review and editing. All authors have read and approved the final version of the manuscript.
Author ORCIDs
Marcin Piątek https://orcid.org/0000-0002-4968-2861
Monika Stryjak-Bogacka https://orcid.org/0000-0003-2845-9975
Paweł Czachura https://orcid.org/0000-0002-3562-8776
Data availability
The data that support the findings of this study are available in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and in culture collections and fungal herbarium, as shown in Table 1 and the text.
Supplementary materials
Phylogenetic tree of selected members of the Capnodiales s. lat., Dothideales and Myriangiales obtained from a maximum likelihood analysis of the combined multi-locus alignment (ITS, LSU, SSU, rpb2, tef1)
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcin Piątek, Monika Stryjak-Bogacka, Paweł Czachura
Data type
tif
Explanation note
The positions of new strains, Arthrocatenaantalyensis comb. nov. and new order Arthrocatenales are indicated in bold. Schismatommadecolorans was used as an outgroup. The scale bar represents the expected number of changes per site.
Phylogenetic tree of selected members of the Capnodiales s. lat., Dothideales and Myriangiales obtained from a Bayesian inference analysis of the combined multi-locus alignment (ITS, LSU, SSU, rpb2, tef1)
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcin Piątek, Monika Stryjak-Bogacka, Paweł Czachura
Data type
tif
Explanation note
The positions of new strains, Arthrocatenaantalyensis comb. nov. and new order Arthrocatenales are indicated in bold. Schismatommadecolorans was used as an outgroup. The scale bar represents the expected number of changes per site.
References
- Abdollahzadeh J, Groenewald JZ, Coetzee MPA, Wingfield MJ, Crous PW. (2020) Evolution of lifestyles in Capnodiales. Studies in Mycology 95: 381–414. 10.1016/j.simyco.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Crous PW, Groenewald JZ. (2008) Devriesiastrelitziae. Fungal Planet, no. 22.
- Avila A, Groenewald JZ, Trapero A, Crous PW. (2005) Characterisation and epitypification of Pseudocercosporacladosporioides, the causal organism of Cercospora leaf spot of olives. Mycological Research 109(8): 881–888. 10.1017/S0953756205003503 [DOI] [PubMed] [Google Scholar]
- Batzer JC, Gleason ML, Harrington TC, Tiffany LH. (2005) Expansion of the sooty blotch and flyspeck complex on apples based on analysis of ribosomal DNA gene sequences and morphology. Mycologia 97(6): 1268–1286. 10.1080/15572536.2006.11832735 [DOI] [PubMed] [Google Scholar]
- Bensch K, Braun U, Groenewald JZ, Crous PW. (2012) The genus Cladosporium. Studies in Mycology 72: 1–401. 10.3114/sim0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra JDP, Sandoval-Denis M, Paiva LM, Silva GA, Groenewald JZ, Souza-Motta CM, Crous PW. (2017) New endophytic Toxicocladosporium species from cacti in Brazil, and description of Neocladosporium gen. nov. IMA Fungus 8(1): 77–97. 10.5598/imafungus.2017.08.01.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose T, Reynolds DR, Berbee ML. (2014) Common, unsightly and until now undescribed: Fumiglobuspieridicola sp. nov., a sooty mold infesting Pierisjaponica from western North America. Mycologia 106(4): 746–756. 10.3852/13-288 [DOI] [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Summerell BA, Hyde KD, To-Anun C, Crous PW. (2009) Myrtaceae, a cache of fungal biodiversity. Persoonia 23(1): 55–85. 10.3767/003158509X474752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Hyde KD, To-anun C, Crous PW. (2012) Chocolate spot disease of Eucalyptus. Mycological Progress 11(1): 61–69. 10.1007/s11557-010-0728-8 [DOI] [Google Scholar]
- Chomnunti P, Schoch CL, Aguirre-Hudson B, Ko-Ko TW, Hongsanan S, Jones EB, Kodsueb R, Phookamsak R, Chukeatirote E, Bahkali AH, Hyde KD. (2011) Capnodiaceae. Fungal Diversity 51(1): 103–134. 10.1007/s13225-011-0145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, Alias AS, Xu J, Liu XH, Stadler M, Hyde KD. (2014) The sooty moulds. Fungal Diversity 66(1): 1–36. 10.1007/s13225-014-0278-5 [DOI] [Google Scholar]
- Cox J, Stone T, Ryan P, Burkle J, Jandarov R, Mendell MJ, Niemeier-Walsh C, Reponen T. (2022) Residential bacteria and fungi identified by high-throughput sequencing and childhood respiratory health. Environmental Research 204(Part D): 112377. 10.1016/j.envres.2021.112377 [DOI] [PubMed]
- Crous PW, Groenewald JZ. (2016) They seldom occur alone. Fungal Biology 120(11): 1392–1415. 10.1016/j.funbio.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ. (2004) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ. (2006) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. 10.3114/sim.55.1.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007a) Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. 10.3114/sim.2007.58.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ. (2007b) Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56. 10.3114/sim.2007.58.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie A, Mohammed C, Himaman W, Groenewald JZ. (2007c) Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus. Fungal Diversity 26: 143–185. [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ. (2008) Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20(1): 59–86. 10.3767/003158508X323949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ. (2009a) Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22(1): 38–48. 10.3767/003158509X424333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ. (2009b) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47S7. 10.3114/sim.2009.64.02 [DOI] [PMC free article] [PubMed]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ. (2009c) Unravelling Mycosphaerella: Do you believe in genera? Persoonia 23(1): 99–118. 10.3767/003158509X479487 [DOI] [PMC free article] [PubMed]
- Crous PW, Groenewald JZ, Pascoe IG, Edwards J. (2010) Devriesiaxanthorrhoeae. Fungal Planet 67. Persoonia 25: 154–155. [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, Edwards J, Seifert KA, Alfenas AC, Alfenas RF, Burgess TI, Carnegie AJ, Hardy GE, Hiscock N, Hüberli D, Jung T, Louis-Seize G, Okada G, Pereira OL, Stukely MJ, Wang W, White GP, Young AJ, McTaggart AR, Pascoe IG, Porter IJ, Quaedvlieg W. (2011a) Fungal Planet description sheets: 69–91. Persoonia 26(1): 108–156. 10.3767/003158511X581723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, Romberg M, Mel’nik VA, Verkley GJ, Groenewald JZ. (2011b) Fungal Planet description sheets: 92–106. Persoonia 27(1): 130–162. 10.3767/003158511X617561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Wingfield MJ, Summerell BA, Rossman AY, Alves JL, Adams GC, Barreto RW, Bell A, Coutinho ML, Flory SL, Gates G, Grice KR, Hardy GE, Kleczewski NM, Lombard L, Longa CM, Louis-Seize G, Macedo F, Mahoney DP, Maresi G, Martin-Sanchez PM, Marvanová L, Minnis AM, Morgado LN, Noordeloos ME, Phillips AJ, Quaedvlieg W, Ryan PG, Saiz-Jimenez C, Seifert KA, Swart WJ, Tan YP, Tanney JB, Thu PQ, Videira SI, Walker DM, Groenewald JZ. (2012) Fungal Planet description sheets: 128–153. Persoonia 29(1): 146–201. 10.3767/003158512X661589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, Shin HD, Nakashima C, Groenewald JZ. (2013a) Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. 10.3114/sim0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, Cheewangkoon R, van der Bank M, Swart WJ, Stchigel AM, Cano-Lira JF, Roux J, Madrid H, Damm U, Wood AR, Shuttleworth LA, Hodges CS, Munster M, de Jesús Yáñez-Morales M, Zúñiga-Estrada L, Cruywagen EM, De Hoog GS, Silvera C, Najafzadeh J, Davison EM, Davison PJN, Barrett MD, Barrett RL, Manamgoda DS, Minnis AM, Kleczewski NM, Flory SL, Castlebury LA, Clay K, Hyde KD, Maússe-Sitoe SND, Chen S, Lechat C, Hairaud M, Lesage-Meessen L, Pawłowska J, Wilk M, Śliwińska-Wyrzychowska A, Mętrak M, Wrzosek M, Pavlic-Zupanc D, Maleme HM, Slippers B, Mac Cormack WP, Archuby DI, Grünwald NJ, Tellería MT, Dueñas M, Martín MP, Marincowitz S, de Beer ZW, Perez CA, Gené J, Marin-Felix Y, Groenewald JZ. (2013b) Fungal Planet description sheets: 154–213. Persoonia 31(1): 188–296. 10.3767/003158513X675925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Schumacher RK, Summerell BA, Giraldo A, Gené J, Guarro J, Wanasinghe DN, Hyde KD, Camporesi E, Gareth Jones EB, Thambugala KM, Malysheva EF, Malysheva VF, Acharya K, Álvarez J, Alvarado P, Assefa A, Barnes CW, Bartlett JS, Blanchette RA, Burgess TI, Carlavilla JR, Coetzee MP, Damm U, Decock CA, den Breeÿen A, de Vries B, Dutta AK, Holdom DG, Rooney-Latham S, Manjón JL, Marincowitz S, Mirabolfathy M, Moreno G, Nakashima C, Papizadeh M, Shahzadeh Fazeli SA, Amoozegar MA, Romberg MK, Shivas RG, Stalpers JA, Stielow B, Stukely MJ, Swart WJ, Tan YP, Vanderbank M, Wood AR, Zhang Y, Groenewald JZ. (2014) Fungal Planet description sheets: 281–319. Persoonia 33(1): 212–289. 10.3767/003158514X685680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MT, Stchigel AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengol J, Barnes I, Cano-Lira JF, Castañeda Ruiz RF, Contu M, Courtecuisse PR, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering AD, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He XL, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat C, Lopes UP, Madrid H, Malysheva EF, Marín-Felix Y, Martín MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Rodas Peláez CA, Rooney-Latham S, Sandoval-Denis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner HG, Wong PT, Wood AR, Groenewald JZ. (2015) Fungal Planet description sheets: 320–370. Persoonia 34(1): 167–266. 10.3767/003158515X688433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, Le Roux JJ, Strasberg D, Edwards J, Roets F, Hubka V, Taylor PW, Heykoop M, Martín MP, Moreno G, Sutton DA, Wiederhold NP, Barnes CW, Carlavilla JR, Gené J, Giraldo A, Guarnaccia V, Guarro J, Hernández-Restrepo M, Kolařík M, Manjón JL, Pascoe IG, Popov ES, Sandoval-Denis M, Woudenberg JH, Acharya K, Alexandrova AV, Alvarado P, Barbosa RN, Baseia IG, Blanchette RA, Boekhout T, Burgess TI, Cano-Lira JF, Čmoková A, Dimitrov RA, Dyakov MY, Dueñas M, Dutta AK, Esteve-Raventós F, Fedosova AG, Fournier J, Gamboa P, Gouliamova DE, Grebenc T, Groenewald M, Hanse B, Hardy GE, Held BW, Jurjević Ž, Kaewgrajang T, Latha KP, Lombard L, Luangsa-Ard JJ, Lysková P, Mallátová N, Manimohan P, Miller AN, Mirabolfathy M, Morozova OV, Obodai M, Oliveira NT, Ordóñez ME, Otto EC, Paloi S, Peterson SW, Phosri C, Roux J, Salazar WA, Sánchez A, Sarria GA, Shin HD, Silva BD, Silva GA, Smith MT, Souza-Motta CM, Stchigel AM, Stoilova-Disheva MM, Sulzbacher MA, Telleria MT, Toapanta C, Traba JM, Valenzuela-Lopez N, Watling R, Groenewald JZ. (2016) Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. 10.3767/003158516X692185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, Hardy GESJ, Barber PA, Alvarado P, Barnes CW, Buchanan PK, Heykoop M, Moreno G, Thangavel R, van der Spuy S, Barili A, Barrett S, Cacciola SO, Cano-Lira JF, Crane C, Decock C, Gibertoni TB, Guarro J, Guevara-Suarez M, Hubka V, Kolařík M, Lira CRS, Ordoñez ME, Padamsee M, Ryvarden L, Soares AM, Stchigel AM, Sutton DA, Vizzini A, Weir BS, Acharya K, Aloi F, Baseia IG, Blanchette RA, Bordallo JJ, Bratek Z, Butler T, Cano-Canals J, Carlavilla JR, Chander J, Cheewangkoon R, Cruz RHSF, da Silva M, Dutta AK, Ercole E, Escobio V, Esteve-Raventós F, Flores JA, Gené J, Góis JS, Haines L, Held BW, Jung MH, Hosaka K, Jung T, Jurjević Ž, Kautman V, Kautmanova I, Kiyashko AA, Kozanek M, Kubátová A, Lafourcade M, La Spada F, Latha KPD, Madrid H, Malysheva EF, Manimohan P, Manjón JL, Martín MP, Mata M, Merényi Z, Morte A, Nagy I, Normand AC, Paloi S, Pattison N, Pawłowska J, Pereira OL, Petterson ME, Picillo B, Raj KNA, Roberts A, Rodríguez A, Rodríguez-Campo FJ, Romański M, Ruszkiewicz-Michalska M, Scanu B, Schena L, Semelbauer M, Sharma R, Shouche YS, Silva V, Staniaszek-Kik M, Stielow JB, Tapia C, Taylor PWJ, Toome-Heller M, Vabeikhokhei JMC, van Diepeningen AD, Van Hoa N. (2017) Fungal Planet description sheets: 558–624. Persoonia 38(1): 240–384. 10.3767/003158517X698941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, Thangavel R, Hernández-Restrepo M, Carnegie AJ, Cheewangkoon R, Wingfield MJ, Summerell BA, Quaedvlieg W, Coutinho TA, Roux J, Wood AR, Giraldo A, Groenewald JZ. (2019a) New and interesting fungi. 2. Fungal Systematics and Evolution 3(1): 57–134. 10.3114/fuse.2019.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Houbraken J. (2019b) Fungal biodiversity, 2nd edition. Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands.
- Crous PW, Costa MM, Kandemir H, Vermaas M, Vu D, Zhao L, Abell SE, Arumugam E, Flakus A, Jurjević Ž, Kaliyaperumal M, Mahadevakumar S, Marney TS, Murugadoss R, Shivas RG, Tan YP, Wingfield MJ, Danteswari C, Darmostuk V, Denchev TT, Etayo J, Gené J, Gunaseelan S, Hubka V, Illescas T, Jansen GM, Kezo K, Kumar S, Larsson E, Mufeeda KT, Piątek M, Rodriguez-Flakus P, Sarma PVSRN, Stryjak-Bogacka M, Torres-Garcia D, Vauras J, Acal DA, Akulov A, Alhudaib K, Asif M, Balashov S, Baral HO, Baturo-Cieśniewska A, Begerow D, Beja-Pereira A, Bianchinotti MV, Bilański P, Chandranayaka S, Chellappan N, Cowan DA, Custódio FA, Czachura P, Delgado G, De Silva NI, Dijksterhuis J, Dueñas M, Eisvand P, Fachada V, Fournier J, Fritsche Y, Fuljer F, Ganga KGG, Guerra MP, Hansen K, Hywel-Jones N, Ismail AM, Jacobs CR, Jankowiak R, Karich A, Kemler M, Kisło K, Klofac W, Krisai-Greilhuber I, Latha KPD, Lebeuf R, Lopes ME, Lumyong S, Maciá-Vicente JG, Maggs-Kölling G, Magistà D, Manimohan P, Martín MP, Mazur E, Mehrabi-Koushki M, Miller AN, Mombert A, Ossowska EA, Patejuk K, Pereira OL, Piskorski S, Plaza M, Podile AR, Polhorský A, Pusz W, Raza M, Ruszkiewicz-Michalska M, Saba M, Sánchez RM, Singh R, Śliwa L, Smith ME, Stefenon VM, Strašiftáková D, Suwannarach N, Szczepańska K, Telleria MT, Tennakoon DS, Thines M, Thorn RG, Urbaniak J, van der Vegte M, Vasan V, Vila-Viçosa C, Voglmayr H, Wrzosek M, Zappelini J, Groenewald JZ. (2023a) Fungal Planet description sheets: 1550–1613. Persoonia 51(1): 280–417. 10.3767/persoonia.2023.51.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Osieck ER, Shivas RG, Tan YP, Bishop-Hurley SL, Esteve-Raventós F, Larsson E, Luangsa-ard JJ, Pancorbo F, Balashov S, Baseia IG, Boekhout T, Chandranayaka S, Cowan DA, Cruz RHSF, Czachura P, De la Peña-Lastra S, Dovana F, Drury B, Fell J, Flakus A, Fotedar R, Jurjević Ž, Kolecka A, Mack J, Maggs-Kölling G, Mahadevakumar S, Mateos A, Mongkolsamrit S, Noisripoom W, Plaza M, Overy DP, Piątek M, Sandoval-Denis M, Vauras J, Wingfield MJ, Abell SE, Ahmadpour A, Akulov A, Alavi F, Alavi Z, Altés A, Alvarado P, Anand G, Ashtekar N, Assyov B, Banc-Prandi G, Barbosa KD, Barreto GG, Bellanger JM, Bezerra JL, Bhat DJ, Bilański P, Bose T, Bozok F, Chaves J, Costa-Rezende DH, Danteswari C, Darmostuk V, Delgado G, Denman S, Eichmeier A, Etayo J, Eyssartier G, Faulwetter S, Ganga KGG, Ghosta Y, Goh J, Góis JS, Gramaje D, Granit L, Groenewald M, Gulden G, Gusmão LFP, Hammerbacher A, Heidarian Z, Hywel-Jones N, Jankowiak R, Kaliyaperumal M, Kaygusuz O, Kezo K, Khonsanit A, Kumar S, Kuo CH, Læssøe T, Latha KPD, Loizides M, Luo SM, Maciá-Vicente JG, Manimohan P, Marbach PAS, Marinho P, Marney TS, Marques G, Martín MP, Miller AN, Mondello F, Moreno G, Mufeeda KT, Mun HY, Nau T, Nkomo T, Okrasińska A, Oliveira JPAF, Oliveira RL, Ortiz DA, Pawłowska J, Pérez-De-Gregorio MÀ, Podile AR, Portugal A, Privitera N, Rajeshkumar KC, Rauf I, Rian B, Rigueiro-Rodríguez A, Rivas-Torres GF, Rodriguez-Flakus P, Romero-Gordillo M, Saar I, Saba M, Santos CD, Sarma PVSRN, Siquier JL, Sleiman S, Spetik M, Sridhar KR, Stryjak-Bogacka M, Szczepańska K, Taşkın H, Tennakoon DS, Thanakitpipattana D, Trovão J, Türkekul İ, van Iperen AL, van ’t Hof P, Vasquez G, Visagie CM, Wingfield BD, Wong PTW, Yang WX, Yarar M, Yarden O, Yilmaz N, Zhang N, Zhu YN, Groenewald JZ. (2023b) Fungal Planet description sheets: 1478–1549. Persoonia 50(1): 158–310. 10.3767/persoonia.2023.50.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachura P, Owczarek-Kościelniak M, Piątek M. (2021) Salinomycespolonicus: A moderately halophilic kin of the most extremely halotolerant fungus Hortaeawerneckii. Fungal Biology 125(6): 459–468. 10.1016/j.funbio.2021.01.003 [DOI] [PubMed] [Google Scholar]
- Darriba D, Posada D, Kozlov AM, Stamatakis A, Morel B, Flouri T. (2020) ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution 37(1): 291–294. 10.1093/molbev/msz189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruyter J, Aveskamp MM, Woudenberg JH, Verkley GJ, Groenewald JZ, Crous PW. (2009) Molecular phylogeny of Phoma and allied anamorph genera: Towards a reclassification of the Phoma complex. Mycological Research 113(4): 508–519. 10.1016/j.mycres.2009.01.002 [DOI] [PubMed] [Google Scholar]
- De Hoog S, Zalar P, Urzì C, De Leo F, Yurlova N, Sterflinger K. (1999) Relationships of dothideaceous black yeasts and meristematic fungi based on 5.8S and ITS2 rDNA sequence comparison. Studies in Mycology 43: 31–37. [Google Scholar]
- De Leo F, Urzì C, de Hoog G. (2003) A new meristematic fungus, Pseudotaeniolinaglobosa. Antonie van Leeuwenhoek 83(4): 351–360. 10.1023/A:1023331502345 [DOI] [PubMed] [Google Scholar]
- De Leo F, Marchetta A, Urzì C. (2022) Black fungi on stone-built heritage: Current knowledge and future outlook. Applied Sciences (Basel, Switzerland) 12(8): 3969. 10.3390/app12083969 [DOI] [Google Scholar]
- Delgado G, Miller A, Piepenbring M. (2018) South Florida microfungi: Castanedospora, a new genus to accommodate Sporidesmiumpachyanthicola (Capnodiales, Ascomycota). Cryptogamie. Mycologie 39(1): 109–127. 10.7872/crym/v39.iss1.2018.109 [DOI] [Google Scholar]
- Dhami MK, Weir BS, Taylor MW, Beggs JR. (2013) Diverse honeydew-consuming fungal communities associated with scale insects. PLoS One 8(7): e70316. 10.1371/journal.pone.0070316 [DOI] [PMC free article] [PubMed]
- Díaz Arias MM, Batzer JC, Harrington TC, Wong AW, Bost SC, Cooley DR, Ellis MA, Hartman JR, Rosenberger DA, Sundin GW, Sutton TB, Travis JW, Wheeler MJ, Yoder KS, Gleason ML. (2010) Diversity and biogeography of sooty blotch and flyspeck fungi on apple in the eastern and midwestern United States. Phytopathology 100(4): 345–355. 10.1094/PHYTO-100-4-0345 [DOI] [PubMed] [Google Scholar]
- Doña J, Proctor H, Serrano D, Johnson KP, Oploo AO, Huguet-Tapia JC, Ascunce MS, Jovani R. (2019) Feather mites play a role in cleaning host feathers: New insights from DNA metabarcoding and microscopy. Molecular Ecology 28(2): 203–218. 10.1111/mec.14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte APM, Attili-Angelis D, Baron NC, Groenewald JZ, Crous PW, Pagnocca FC. (2017) Riding with the ants. Persoonia 38(1): 81–99. 10.3767/003158517X693417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egidi E, de Hoog GS, Isola D, Onofri S, Quaedvlieg W, de Vries M, Verkley GJM, Stielow JB, Zucconi L, Selbmann L. (2014) Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Diversity 65(1): 127–165. 10.1007/s13225-013-0277-y [DOI] [Google Scholar]
- Fan XL, Barreto RW, Groenewald JZ, Bezerra JD, Pereira OL, Cheewangkoon R, Mostert L, Tian CM, Crous PW. (2017) Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Studies in Mycology 87(1): 1–41. 10.1016/j.simyco.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flessa F, Rambold G. (2013) Diversity of the Capnocheiridesrhododendri-dominated fungal community in the phyllosphere of Rhododendronferrugineum L. Nova Hedwigia 97(1–2): 19–53. 10.1127/0029-5035/2013/0110 [DOI] [Google Scholar]
- Flessa F, Peršoh D, Rambold G. (2012) Annuality of Central European deciduous tree leaves delimits community development of epifoliar pigmented fungi. Fungal Ecology 5(5): 554–561. 10.1016/j.funeco.2011.12.005 [DOI] [Google Scholar]
- Flessa F, Harjes J, Cáceres MES, Rambold G. (2021) Comparative analyses of sooty mould communities from Brazil and Central Europe. Mycological Progress 20(7): 869–887. 10.1007/s11557-021-01700-0 [DOI] [Google Scholar]
- Frank J, Crous PW, Groenewald JZ, Oertel B, Hyde KD, Phengsintham P, Schroers HJ. (2010) Microcyclospora and Microcyclosporella: Novel genera accommodating epiphytic fungi causing sooty blotch on apple. Persoonia 24(1): 93–105. 10.3767/003158510X510560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend RJ. (1965) A study of sooty mould on lime trees (Tilia×vulgaris). Transactions of the British Mycological Society 48(3): 367–370. 10.1016/S0007-1536(65)80056-0 [DOI] [Google Scholar]
- Giampetruzzi A, Baptista P, Morelli M, Cameirão C, Lino Neto T, Costa D, D’Attoma G, Abou Kubaa R, Altamura G, Saponari M, Pereira JA, Saldarelli P. (2020) Differences in the endophytic microbiome of olive cultivars infected by Xylellafastidiosa across seasons. Pathogens (Basel, Switzerland) 9(9): 723. 10.3390/pathogens9090723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason ML, Zhang R, Batzer JC, Sun G. (2019) Stealth pathogens: The sooty blotch and flyspeck fungal complex. Annual Review of Phytopathology 57(1): 135–164. 10.1146/annurev-phyto-082718-100237 [DOI] [PubMed] [Google Scholar]
- Groenewald M, Groenewald JZ, Crous PW. (2005) Distinct species exist within the Cercosporaapii morphotype. Phytopathology 95(8): 951–959. 10.1094/PHYTO-95-0951 [DOI] [PubMed] [Google Scholar]
- Groenewald JZ, Nakashima C, Nishikawa J, Shin HD, Park JH, Jama AN, Groenewald M, Braun U, Crous PW. (2013) Species concepts in Cercospora: Spotting the weeds among the roses. Studies in Mycology 75(1): 115–170. 10.3114/sim0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S, Tsuneda A, Currah RS. (2003) Comparative morphology and phylogenetic placement of two microsclerotial black fungi from Sphagnum. Mycologia 95(5): 959–975. 10.1080/15572536.2004.11833055 [DOI] [PubMed] [Google Scholar]
- Hongsanan S, Hyde KD, Bahkali AH, Camporesi E, Chomnunti P, Ekanayaka H, Gomes AAM, Hofstetter V, Jones EBG, Pinho DB, Pereira OL, Tian Q, Wanasinghe DN, Xu JC, Buyck B. (2015a) Fungal Biodiversity Profiles 11–20. Cryptogamie. Mycologie 2015(3): 355–380. 10.7872/crym/v36.iss3.2015.355 [DOI] [Google Scholar]
- Hongsanan S, Tian Q, Hyde KD, Chomnunti P. (2015b) Two new species of sooty moulds, Capnodiumcoffeicola and Conidiocarpusplumeriae in Capnodiaceae. Mycosphere: Journal of Fungal Biology 6(6): 814–824. 10.5943/mycosphere/6/6/14 [DOI] [Google Scholar]
- Hongsanan S, Zhao RL, Hyde KD. (2017) A new species of Chaetothyrina on branches of mango, and introducing Phaeothecoidiellaceae fam. nov. Mycosphere: Journal of Fungal Biology 8(1): 137–146. 10.5943/mycosphere/8/1/13 [DOI] [Google Scholar]
- Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Bhat DJ, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe IS, Tibpromma S, Jayasiri SC, Sandamali DS, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Chethana KWT, Samarakoon MC, Ertz D, Bao DF, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, Norphanphoun C, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-González R, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Aluthmuhandiram JVS, Abeywickrama PD, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Suetrong S, Chaiwan N, Dayarathne MC, Yang J, Rathnayaka AR, Bhunjun CS, Xu JC, Zheng JS, Liu G, Feng Y, Xie N. (2020) Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere: Journal of Fungal Biology 11(1): 1553–2107. 10.5943/mycosphere/11/1/13 [DOI] [Google Scholar]
- Hughes SJ. (1976) Sooty moulds. Mycologia 68(4): 693–820. 10.1080/00275514.1976.12019958 [DOI] [Google Scholar]
- Hughes SJ, Seifert KA. (2012) Taxonomic and nomenclatural notes on sooty mould names based on species mixtures: Hormisciumhandelii and Torulalechleriana. Mycoscience 53(1): 17–24. 10.1007/s10267-011-0133-4 [DOI] [Google Scholar]
- Hujslová M, Kubátová A, Kostovčík M, Kolařík M. (2013) Acidiellabohemica gen. et sp. nov. and Acidomyces spp. (Teratosphaeriaceae), the indigenous inhabitants of extremely acidic soils in Europe. Fungal Diversity 58(1): 33–45. 10.1007/s13225-012-0176-7 [DOI] [Google Scholar]
- Hunter GC, Wingfield BD, Crous PW, Wingfield MJ. (2006) A multi-gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Studies in Mycology 55: 147–161. 10.3114/sim.55.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail SI, Batzer JC, Harrington TC, Crous PW, Lavrov DV, Li H, Gleason ML. (2016) Ancestral state reconstruction infers phytopathogenic origins of sooty blotch and flyspeck fungi on apple. Mycologia 108(2): 292–302. 10.3852/15-036 [DOI] [PubMed] [Google Scholar]
- Isola D, Zucconi L, Onofri S, Caneva G, de Hoog GS, Selbmann L. (2016) Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Diversity 76(1): 75–96. 10.1007/s13225-015-0342-9 [DOI] [Google Scholar]
- Jacobs KA, Rehner SA. (1998) Comparison of cultural and morphological characters and ITS sequences in anamorphs of Botryosphaeria and related taxa. Mycologia 90(4): 601–610. 10.1080/00275514.1998.12026949 [DOI] [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O’Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüssler A, Longcore JE, O’Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KW, Matsuura K, Langer E, Langer G, Untereiner WA, Lücking R, Büdel B, Geiser DM, Aptroot A, Diederich P, Schmitt I, Schultz M, Yahr R, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R. (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443(7113): 818–822. 10.1038/nature05110 [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. (2005) MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33(2): 511–518. 10.1093/nar/gki198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Choi JH, Nam SH, Fyumagwa R, Yong TS. (2022) Parasites and blood-meal hosts of the tsetse fly in Tanzania: A metagenomics study. Parasites & Vectors 15(1): 224. 10.1186/s13071-022-05344-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. (2019) RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics (Oxford, England) 35(21): 4453–4455. 10.1093/bioinformatics/btz305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küngas K, Bahram M, Põldmaa K. (2020) Host tree organ is the primary driver of endophytic fungal community structure in a hemiboreal forest. FEMS Microbiology Ecology 96(2): fiz199. 10.1093/femsec/fiz199 [DOI] [PubMed]
- Laichmanová M. (2023) Taxonomy of rock-inhabiting fungi from James Ross Island, Antarctica. Czech Polar Reports 13(1): 81–95. 10.5817/CPR2023-1-8 [DOI] [Google Scholar]
- Lee SY, Lim YS, Jung HY. (2016) Molecular phylogeny and morphology of Mycosphaerellanawae, the causal agent of circular leaf spot on persimmon. Mycobiology 44(4): 191–201. 10.5941/MYCO.2016.44.4.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Sun GY, Zhai XR, Batzer JC, Mayfield DA, Crous PW, Groenewald JZ, Gleason ML. (2012) Dissoconiaceae associated with sooty blotch and flyspeck on fruits in China and the United States. Persoonia 28(1): 113–125. 10.3767/003158512X651157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wu H-X, Li J, Chen H, Wang W. (2020) The insights into the evolutionary history of Translucidithyrium: Based on a newly-discovered species. MycoKeys 76: 1–16. 10.3897/mycokeys.76.58628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16(12): 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SNN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, D’souza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe D, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E. (2015) Fungal diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Diversity 72(1): 1–197. 10.1007/s13225-015-0324-y [DOI] [Google Scholar]
- Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung GH, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim YW, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R. (2004) Assembling the fungal tree of life: Progress, classification, and evolution of subcellular traits. American Journal of Botany 91(10): 1446–1480. 10.3732/ajb.91.10.1446 [DOI] [PubMed] [Google Scholar]
- Minnis AM, Rossman AY, Olsen RT. (2011) Mycosphaerellanyssicola revisited: A species distinct from M.punctiformis. Mycotaxon 115(1): 311–322. 10.5248/115.311 [DOI] [Google Scholar]
- Muggia L, Grube M. (2010) Fungal composition of lichen thalli assessed by single strand conformation polymorphism. Lichenologist (London, England) 42(4): 461–473. 10.1017/S0024282909990752 [DOI] [Google Scholar]
- Muggia L, Hafellner J, Wirtz N, Hawksworth DL, Grube M. (2008) The sterile microfilamentous lichenized fungi Cystocoleusebeneus and Racodiumrupestre are relatives of plant pathogens and clinically important dothidealean fungi. Mycological Research 112(1): 50–56. 10.1016/j.mycres.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Pereira DRS, Phillips AJL. (2020) A new leaf spot disease of Chamaeropshumilis caused by Palmeiromyceschamaeropicola gen. et sp. nov. Phytopathologia Mediterranea 59(2): 353–363. [Google Scholar]
- Piątek M, Stryjak-Bogacka M, Czachura P, Owczarek-Kościelniak M. (2023) The genus Rachicladosporium: Introducing new species from sooty mould communities and excluding cold adapted species. Scientific Reports 13(1): 22795. 10.1038/s41598-023-49696-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Kema GH, Groenewald JZ, Verkley GJ, Seifbarghi S, Razavi M, Mirzadi Gohari A, Mehrabi R, Crous PW. (2011) Zymoseptoria gen. nov.: A new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26(1): 57–69. 10.3767/003158511X571841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJ, Shin HD, Barreto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW. (2013) Sizing up Septoria. Studies in Mycology 75(1): 307–390. 10.3114/sim0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, Summerell BA, Carnegie AJ, Burgess TI, Crous PW. (2014) Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33(1): 1–40. 10.3767/003158514X681981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley AW. (1996) Comminutispora gen. nov. and its Hyphospora gen. nov. anamorph. Mycologia 88(1): 132–136. 10.1080/00275514.1996.12026633 [DOI] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal C, Platas G, Bills GF. (2005) Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycological Progress 4(1): 23–38. 10.1007/s11557-006-0107-7 [DOI] [Google Scholar]
- Ruibal C, Platas G, Bills GF. (2008) High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 21(1): 93–110. 10.3767/003158508X371379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, Groenewald JZ, Muggia L, Grube M, Isola D, Schoch CL, Staley JT, Lutzoni F, de Hoog GS. (2009) Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Studies in Mycology 64: 123–133S7. 10.3114/sim.2009.64.06 [DOI] [PMC free article] [PubMed]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98(6): 1041–1052. 10.1080/15572536.2006.11832632 [DOI] [PubMed] [Google Scholar]
- Schoch CL, Kohlmeyer J, Volkmann-Kohlmeyer B, Tsui CK, Spatafora JW. (2006a) The halotolerant fungus Glomerobolusgelineus is a member of the Ostropales. Mycological Research 110(3): 257–263. 10.1016/j.mycres.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EW, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EB, Kohlmeyer J, Kruys A, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJ, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Plata ER, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JH, Yonezawa H, Zhang Y, Spatafora JW. (2009) A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15S10. 10.3114/sim.2009.64.01 [DOI] [PMC free article] [PubMed]
- Schubert K, Braun U, Groenewald JZ, Crous PW. (2007) Cladosporium leaf-blotch and stem rot of Paeonia spp. caused by Dichocladosporiumchlorocephalum gen. nov. Studies in Mycology 58: 95–104. 10.3114/sim.2007.58.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Untereiner WA, Ewaze JO, Wong B, Doyle D. (2007) Baudoinia, a new genus to accommodate Torulacompniacensis. Mycologia 99(4): 592–601. 10.1080/15572536.2007.11832553 [DOI] [PubMed] [Google Scholar]
- Sert H, Sümbül H, Sterflinger K. (2007) A new species of Capnobotryella from monument surfaces. Mycological Research 111(10): 1235–1241. 10.1016/j.mycres.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Simon UK, Groenewald JZ, Crous PW. (2009) Cymadotheatrifolii, an obligate biotrophic leaf parasite of Trifolium, belongs to Mycosphaerellaceae as shown by nuclear ribosomal DNA analyses. Persoonia 22(1): 49–55. 10.3767/003158509X425350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singtripop D, Hongsanan S, Li J, Indeewari de Silva N, Phillips AJL, Jones EBG, Bahkali AH, Hyde KD. (2016) Chaetothyrinamangiferae sp. nov., a new species of Chaetothyrina. Phytotaxa 255(1): 21–33. 10.11646/phytotaxa.255.1.2 [DOI] [Google Scholar]
- Spatafora JW, Sung GH, Johnson D, Hesse C, O’Rourke B, Serdani M, Spotts R, Lutzoni F, Hofstetter V, Miadlikowska J, Reeb V, Gueidan C, Fraker E, Lumbsch T, Lücking R, Schmitt I, Hosaka K, Aptroot A, Roux C, Miller AN, Geiser DM, Hafellner J, Hestmark G, Arnold AE, Büdel B, Rauhut A, Hewitt D, Untereiner WA, Cole MS, Scheidegger C, Schultz M, Sipman H, Schoch CL. (2006) A five-gene phylogeny of Pezizomycotina. Mycologia 98(6): 1018–1028. 10.1080/15572536.2006.11832630 [DOI] [PubMed] [Google Scholar]
- Sterflinger K, de Hoog GS, Haase G. (1999) Phylogeny and ecology of meristematic ascomycetes. Studies in Mycology 43: 5–22. [Google Scholar]
- Sugiyama J, Amano N. (1987) Two metacapnodiaceous sooty moulds from Japan: their identity and behavior in pure culture. In: Sugiyama J. (Ed.) Pleomorphic fungi: the diversity and its taxonomic implications.Kodansha, Tokyo, and Elsevier, Amsterdam, 141–156. 10.1016/B978-0-444-98966-6.50011-3 [DOI]
- Titze A, de Hoog GS. (1990) Capnobotryellarenispora on roof tile. Antonie van Leeuwenhoek 58(4): 265–269. 10.1007/BF00399338 [DOI] [PubMed] [Google Scholar]
- Trovão J, Tiago I, Soares F, Paiva DS, Mesquita N, Coelho C, Catarino L, Gil F, Portugal A. (2019) Description of Aeminiaceae fam. nov., Aeminium gen. nov. and Aeminiumludgeri sp. nov. (Capnodiales), isolated from a biodeteriorated art-piece in the Old Cathedral of Coimbra, Portugal. MycoKeys 45: 57–73. 10.3897/mycokeys.45.31799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneda A, Davey ML, Hambleton S, Currah RS. (2008) Endosporium, a new endoconidial genus allied to the Myriangiales. Botany 86(9): 1020–1033. 10.1139/B08-054 [DOI] [Google Scholar]
- Verkley GJ, Quaedvlieg W, Shin HD, Crous PW. (2013) A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305. 10.3114/sim0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SI, Groenewald JZ, Kolecka A, van Haren L, Boekhout T, Crous PW. (2015a) Elucidating the Ramulariaeucalypti species complex. Persoonia 34(1): 50–64. 10.3767/003158515X685670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SI, Groenewald JZ, Verkley GJ, Braun U, Crous PW. (2015b) The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology 119(9): 823–843. 10.1016/j.funbio.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Videira SI, Groenewald JZ, Braun U, Shin HD, Crous PW. (2016) All that glitters is not Ramularia. Studies in Mycology 83(1): 49–163. 10.1016/j.simyco.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, Braun U, Barreto RW, de Wit PJGM, Crous PW. (2017) Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87(1): 257–421. 10.1016/j.simyco.2017.09.003 [DOI] [PMC free article] [PubMed]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 171(8): 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM. (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92(1): 135–154. 10.1016/j.simyco.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR protocols, a guide to methods and applications.Academic Press Inc., San Diego, CA, and London, UK, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, D’souza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu JK, Lücking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wanasinghe DN, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat JD, Gueidan C, Chomnunti P, de Hoog GS, Knudsen K, Li WJ, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Piątek M, Raja HA, Shivas RG, Slippers B, Taylor JE, Tian Q, Wang Y, Woudenberg JHC, Cai L, Jaklitsch WM, Hyde KD. (2014) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Diversity 69(1): 1–55. 10.1007/s13225-014-0309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardene NN, Hyde KD, Tibpromma S, Wanasinghe DN, Thambugala KM, Tian Q, Wang Y. (2017) Towards incorporating asexual fungi in a natural classification: Checklist and notes 2012–2016. Mycosphere: Journal of Fungal Biology 8(9): 1457–1555. 10.5943/mycosphere/8/9/10 [DOI] [Google Scholar]
- Wijayawardene NN, Hyde KD, Dai DQ, Sánchez-García M, Goto BT, Saxena RK, Erdoğdu M, Selçuk F, Rajeshkumar KC, Aptroot A, Błaszkowski J, Boonyuen N, da Silva GA, de Souza FA, Dong W, Ertz D, Haelewaters D, Jones EBG, Karunarathna SC, Kirk PM, Kukwa M, Kumla J, Leontyev DV, Lumbsch HT, Maharachchikumbura SSN, Marguno F, Martínez-Rodríguez P, Mešić A, Monteiro JS, Oehl F, Pawłowska J, Pem D, Pfliegler WP, Phillips AJL, Pošta A, He MQ, Li JX, Raza M, Sruthi OP, Suetrong S, Suwannarach N, Tedersoo L, Thiyagaraja V, Tibpromma S, Tkalčec Z, Tokarev YS, Wanasinghe DN, Wijesundara DSA, Wimalaseana SDMK, Madrid H, Zhang GQ, Gao Y, Sánchez-Castro I, Tang LZ, Stadler M, Yurkov A, Thines M. (2022) Outline of Fungi and fungus-like taxa – 2021. Mycosphere: Journal of Fungal Biology 13(1): 53–453. 10.5943/mycosphere/13/1/2 [DOI] [Google Scholar]
- Winton LM, Stone JK, Hansen EM, Shoemaker RA. (2007) The systematic position of Phaeocryptopusgaeumannii. Mycologia 99(2): 240–252. 10.1080/15572536.2007.11832584 [DOI] [PubMed] [Google Scholar]
- Wu Q, Wei D, Dong L, Liu Y, Ren C, Liu Q, Chen C, Chen J, Pei J. (2019) Variation in the microbial community contributes to the improvement of the main active compounds of Magnoliaofficinalis Rehd. et Wils in the process of sweating. Chinese Medicine 14(1): 45. 10.1186/s13020-019-0267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Sun GY, Batzer JC, Crous PW, Groenewald JZ, Gleason ML. (2010) Novel fungal genera and species associated with the sooty blotch and flyspeck complex on apple in China and the USA. Persoonia 24(1): 29–37. 10.3767/003158510X492101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ariyawansa HA, Wu HX, Hyde KD. (2014) The genus Leptoxyphium (Capnodiaceae) from China. Phytotaxa 176(1): 174–183. 10.11646/phytotaxa.176.1.17 [DOI] [Google Scholar]
- Zalar P, de Hoog GS, Gunde-Cimerman N. (1999) Taxonomy of the endoconidial black yeast genera Phaeotheca and Hyphospora. Studies in Mycology 43: 49–56. [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, Miller W. (2000) A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7(1–2): 203–214. 10.1089/10665270050081478 [DOI] [PubMed] [Google Scholar]
- Zucconi L, Gagliardi M, Isola D, Onofri S, Andaloro MC, Pelosi C, Pogliani P, Selbmann L. (2012) Biodeterioration agents dwelling in or on the wall paintings of the Holy Saviour’s cave (Vallerano, Italy). International Biodeterioration & Biodegradation 70: 40–46. 10.1016/j.ibiod.2011.11.018 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of selected members of the Capnodiales s. lat., Dothideales and Myriangiales obtained from a maximum likelihood analysis of the combined multi-locus alignment (ITS, LSU, SSU, rpb2, tef1)
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcin Piątek, Monika Stryjak-Bogacka, Paweł Czachura
Data type
tif
Explanation note
The positions of new strains, Arthrocatenaantalyensis comb. nov. and new order Arthrocatenales are indicated in bold. Schismatommadecolorans was used as an outgroup. The scale bar represents the expected number of changes per site.
Phylogenetic tree of selected members of the Capnodiales s. lat., Dothideales and Myriangiales obtained from a Bayesian inference analysis of the combined multi-locus alignment (ITS, LSU, SSU, rpb2, tef1)
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcin Piątek, Monika Stryjak-Bogacka, Paweł Czachura
Data type
tif
Explanation note
The positions of new strains, Arthrocatenaantalyensis comb. nov. and new order Arthrocatenales are indicated in bold. Schismatommadecolorans was used as an outgroup. The scale bar represents the expected number of changes per site.
Data Availability Statement
The data that support the findings of this study are available in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and in culture collections and fungal herbarium, as shown in Table 1 and the text.