Abstract
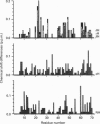
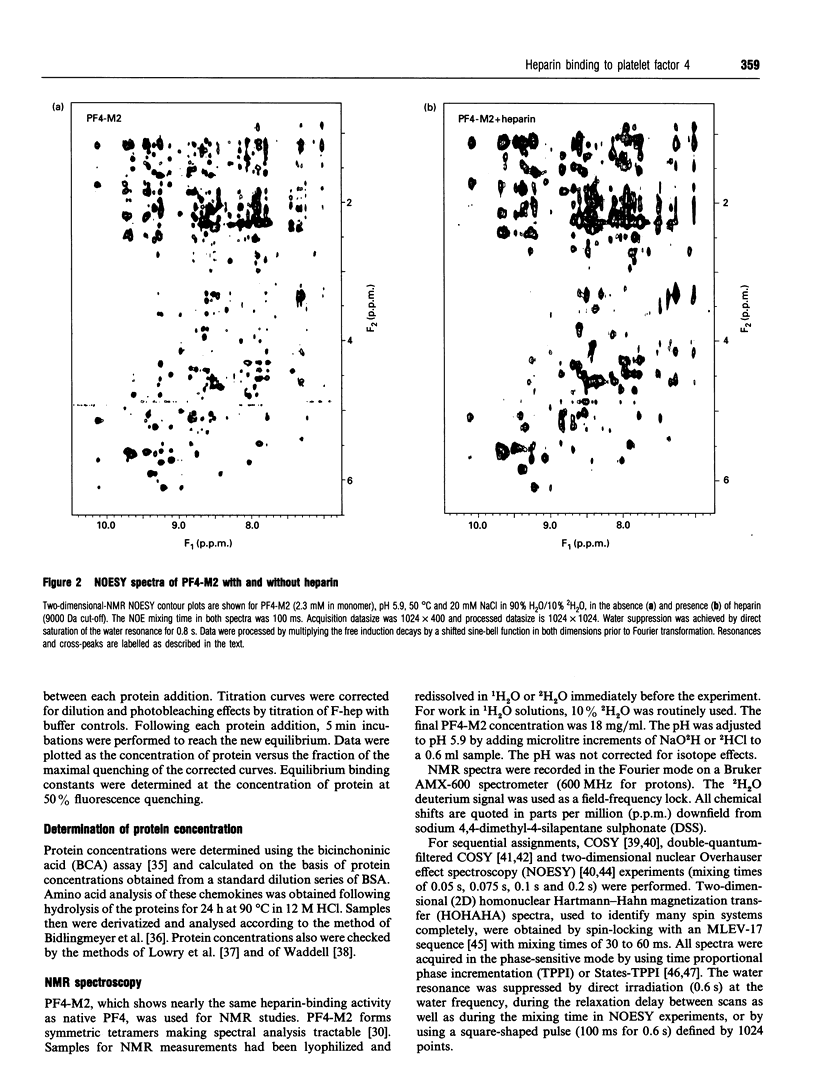
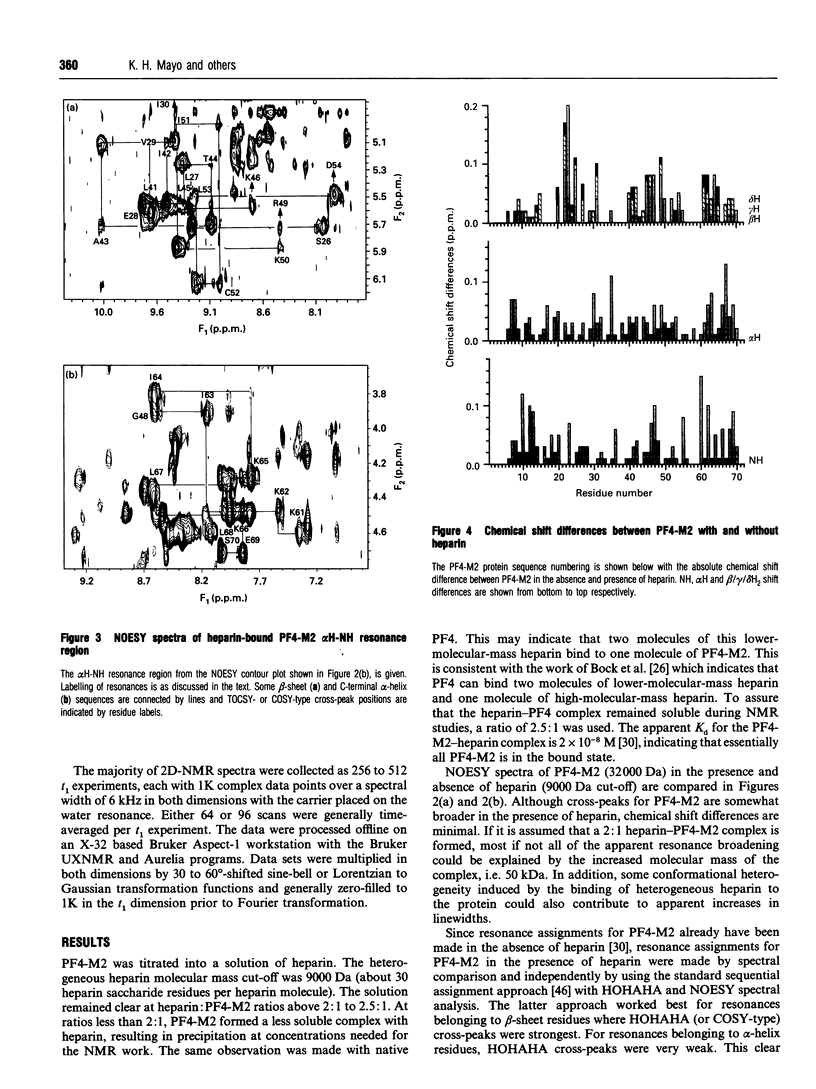
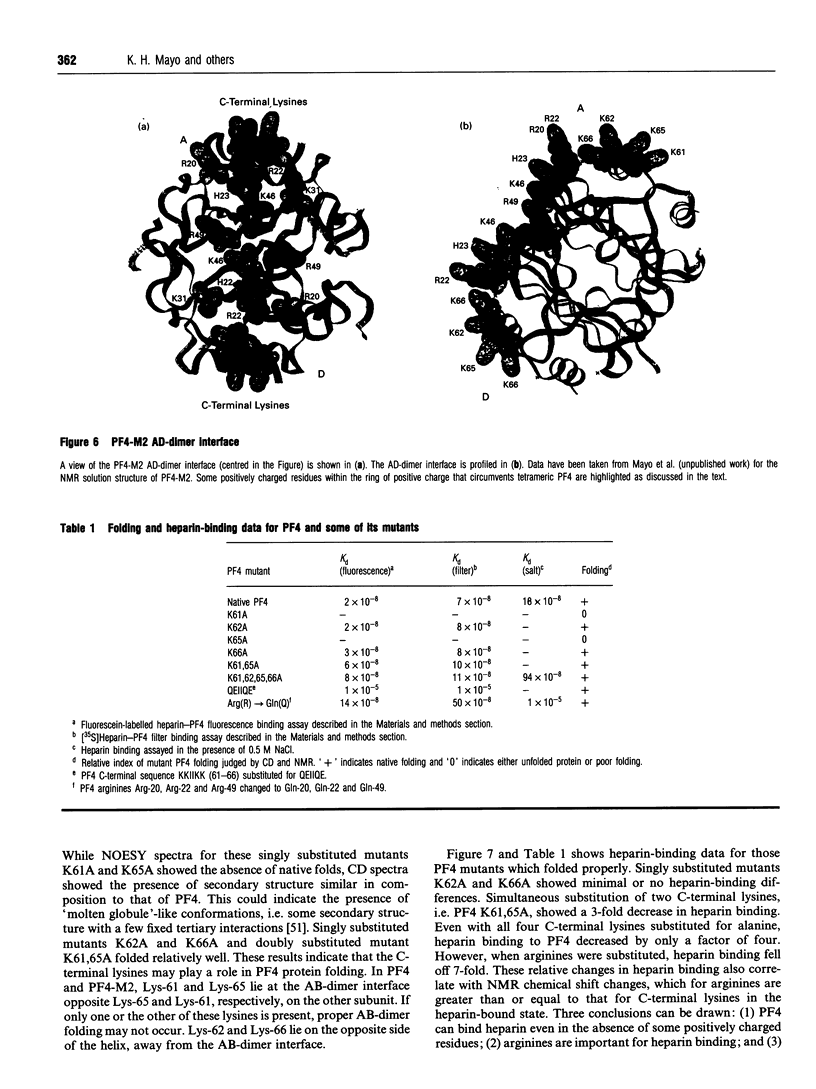
Native platelet factor-4 (PF4) is an asymmetrically associated, homo-tetrameric protein (70 residues/subunit) known for binding polysulphated glycosaminoglycans like heparin. PF4 N-terminal chimeric mutant M2 (PF4-M2), on the other hand, forms symmetric tetramers [Mayo, Roongta, Ilyina, Milius, Barker, Quinlan, La Rosa and Daly (1995) Biochemistry 34, 11399-11409] making NMR studies with this 32 kDa protein tractable. PF4-M2, moreover, binds heparin with a similar affinity to that of native PF4. NMR data presented here indicate that heparin (9000 Da cut-off) binding to PF4-M2, while not perturbing the overall structure of the protein, does perturb specific side-chain proton resonances which map to spatially related residues within a ring of positively charged side chains on the surface of tetrameric PF4-M2. Contrary to PF4-heparin binding models which centre around C-terminal alpha-helix lysines, this study indicates that a loop containing Arg-20, Arg-22, His-23 and Thr-25, as well as Lys-46 and Arg-49, are even more affected by heparin binding. Site-directed mutagenesis and heparin binding data support these NMR findings by indicating that arginines more than C-terminal lysines, are crucial to the heparin binding process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. J., Käser-Glanzmann R., Jakábová M., Lüscher E. F. Characterization of a chondroitin 4 -sulfate proteoglycan carrier for heparin neutralizing activity (platelet factor 4 ) released from human blood platelets. Biochim Biophys Acta. 1972 Dec 29;286(2):312–329. [PubMed] [Google Scholar]
- Bebawy S. T., Gorka J., Hyers T. M., Webster R. O. In vitro effects of platelet factor 4 on normal human neutrophil functions. J Leukoc Biol. 1986 Apr;39(4):423–434. doi: 10.1002/jlb.39.4.423. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bock P. E., Luscombe M., Marshall S. E., Pepper D. S., Holbrook J. J. The multiple complexes formed by the interaction of platelet factor 4 with heparin. Biochem J. 1980 Dec 1;191(3):769–776. doi: 10.1042/bj1910769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley L. L., Sweet J. M., Goetzl E. J. Stimulation of histamine release from human basophils by human platelet factor 4. J Clin Invest. 1983 Oct;72(4):1218–1223. doi: 10.1172/JCI111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio A. M., Niewiarowski S., Rucinski B., Tuszynski G. P., Cierniewski C. S., Hershock D., Kornecki E. Interaction of platelet factor 4 with human platelets. Biochim Biophys Acta. 1985 Apr 17;839(2):161–173. doi: 10.1016/0304-4165(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Appella E., Yamada M., Matsushima K., Gronenborn A. M. Three-dimensional structure of interleukin 8 in solution. Biochemistry. 1990 Feb 20;29(7):1689–1696. doi: 10.1021/bi00459a004. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Bakshi E. N., Machin K. J., Isaacs N. W. Binding of heparin to human platelet factor 4. Biochem J. 1986 Mar 1;234(2):485–488. doi: 10.1042/bj2340485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K., Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991 Oct 29;30(43):10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Keim P. S., Farmer M., Heinrikson R. L. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2256–2258. doi: 10.1073/pnas.74.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson A. E., Cousens L. S., Matthews B. W. Refinement of the structure of human basic fibroblast growth factor at 1.6 A resolution and analysis of presumed heparin binding sites by selenate substitution. Protein Sci. 1993 Aug;2(8):1274–1284. doi: 10.1002/pro.5560020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother W. J., Reilly D., Colby T. J., Hesselgesser J., Horuk R. The solution structure of melanoma growth stimulating activity. J Mol Biol. 1994 Sep 23;242(3):252–270. doi: 10.1006/jmbi.1994.1577. [DOI] [PubMed] [Google Scholar]
- Fujii S., Lucore C. L., Hopkins W. E., Billadello J. J., Sobel B. E. Induction of synthesis of plasminogen activator inhibitor type-1 by tissue-type plasminogen activator in human hepatic and endothelial cells. Thromb Haemost. 1990 Nov 30;64(3):412–419. [PubMed] [Google Scholar]
- Gelman R. A., Blackwell J. Interactions between mucopolysaccharides and cationic polypeptides in aqueous solution: hyaluronic acid, heparitin sulfate, and keratan sulfate. Biopolymers. 1974 Jan;13(1):139–156. doi: 10.1002/bip.1974.360130109. [DOI] [PubMed] [Google Scholar]
- Gelman R. A., Glaser D. N., Blackwell J. Interaction between chondroitin-6-sulfate and poly-L-arginine in aqueous solution. Biopolymers. 1973 Jun;12(6):1223–1232. doi: 10.1002/bip.1973.360120603. [DOI] [PubMed] [Google Scholar]
- Gewirtz A. M., Calabretta B., Rucinski B., Niewiarowski S., Xu W. Y. Inhibition of human megakaryocytopoiesis in vitro by platelet factor 4 (PF4) and a synthetic COOH-terminal PF4 peptide. J Clin Invest. 1989 May;83(5):1477–1486. doi: 10.1172/JCI114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H., Suzuki S., Saito T., Tamura T., Harada T., Yoshida K., Kimata K. Structure of a heparan sulphate oligosaccharide that binds to basic fibroblast growth factor. Biochem J. 1992 Aug 1;285(Pt 3):805–813. doi: 10.1042/bj2850805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z. C., Sensébe L., Abgrall J. F., Brière J. Platelet factor 4 inhibits human megakaryocytopoiesis in vitro. Blood. 1990 Mar 15;75(6):1234–1239. [PubMed] [Google Scholar]
- Handin R. I., Cohen H. J. Purification and binding properties of human platelet factor four. J Biol Chem. 1976 Jul 25;251(14):4273–4282. [PubMed] [Google Scholar]
- Holt J. C., Niewiarowski S. Biochemistry of alpha granule proteins. Semin Hematol. 1985 Apr;22(2):151–163. [PubMed] [Google Scholar]
- Huang S. S., Huang J. S., Deuel T. F. Proteoglycan carrier of human platelet factor 4. Isolation and characterization. J Biol Chem. 1982 Oct 10;257(19):11546–11550. [PubMed] [Google Scholar]
- Ilyina E., Mayo K. H. Multiple native-like conformations trapped via self-association-induced hydrophobic collapse of the 33-residue beta-sheet domain from platelet factor 4. Biochem J. 1995 Mar 1;306(Pt 2):407–419. doi: 10.1042/bj3060407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyina E., Milius R., Mayo K. H. Synthetic peptides probe folding initiation sites in platelet factor-4: stable chain reversal found within the hydrophobic sequence LIATLKNGRKISL. Biochemistry. 1994 Nov 15;33(45):13436–13444. doi: 10.1021/bi00249a032. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991 Apr 2;30(13):3147–3161. doi: 10.1021/bi00227a001. [DOI] [PubMed] [Google Scholar]
- Jeanloz R. W. The chemistry of heparin. Adv Exp Med Biol. 1975;52:3–17. doi: 10.1007/978-1-4684-0946-8_1. [DOI] [PubMed] [Google Scholar]
- Jukes T. H. Arginine as an evolutionary intruder into protein synthesis. Biochem Biophys Res Commun. 1973 Aug 6;53(3):709–714. doi: 10.1016/0006-291x(73)90151-4. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laterra J., Silbert J. E., Culp L. A. Cell surface heparan sulfate mediates some adhesive responses to glycosaminoglycan-binding matrices, including fibronectin. J Cell Biol. 1983 Jan;96(1):112–123. doi: 10.1083/jcb.96.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonky S. A., Wohl H. Stimulation of human leukocyte elastase by platelet factor 4. Physiologic, morphologic, and biochemical effects on hamster lungs in vitro. J Clin Invest. 1981 Mar;67(3):817–826. doi: 10.1172/JCI110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J., Melnick B., Handin R. I. The interaction of platelet factor four and glycosaminoglycans. Arch Biochem Biophys. 1985 Jul;240(1):446–455. doi: 10.1016/0003-9861(85)90049-9. [DOI] [PubMed] [Google Scholar]
- Lyon M., Deakin J. A., Mizuno K., Nakamura T., Gallagher J. T. Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate structural determinants. J Biol Chem. 1994 Apr 15;269(15):11216–11223. [PubMed] [Google Scholar]
- Maccarana M., Casu B., Lindahl U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem. 1993 Nov 15;268(32):23898–23905. [PubMed] [Google Scholar]
- Machovich R., Staub M., Patthy L. Decreased heparin sensitivity of cycholhexanedione-modified thrombin. Eur J Biochem. 1978 Feb;83(2):473–477. doi: 10.1111/j.1432-1033.1978.tb12113.x. [DOI] [PubMed] [Google Scholar]
- Maione T. E., Gray G. S., Petro J., Hunt A. J., Donner A. L., Bauer S. I., Carson H. F., Sharpe R. J. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990 Jan 5;247(4938):77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- Mascotti D. P., Lohman T. M. Thermodynamics of charged oligopeptide-heparin interactions. Biochemistry. 1995 Mar 7;34(9):2908–2915. doi: 10.1021/bi00009a022. [DOI] [PubMed] [Google Scholar]
- Mayo K. H., Chen M. J. Human platelet factor 4 monomer-dimer-tetramer equilibria investigated by 1H NMR spectroscopy. Biochemistry. 1989 Nov 28;28(24):9469–9478. doi: 10.1021/bi00450a034. [DOI] [PubMed] [Google Scholar]
- Mayo K. H. Low-affinity platelet factor 4 1H NMR derived aggregate equilibria indicate a physiologic preference for monomers over dimers and tetramers. Biochemistry. 1991 Jan 29;30(4):925–934. doi: 10.1021/bi00218a007. [DOI] [PubMed] [Google Scholar]
- Mayo K. H., Roongta V., Ilyina E., Milius R., Barker S., Quinlan C., La Rosa G., Daly T. J. NMR solution structure of the 32-kDa platelet factor 4 ELR-motif N-terminal chimera: a symmetric tetramer. Biochemistry. 1995 Sep 12;34(36):11399–11409. doi: 10.1021/bi00036a012. [DOI] [PubMed] [Google Scholar]
- Mayo K. H., Yang Y., Daly T. J., Barry J. K., La Rosa G. J. Secondary structure of neutrophil-activating peptide-2 determined by 1H-nuclear magnetic resonance spectroscopy. Biochem J. 1994 Dec 1;304(Pt 2):371–376. doi: 10.1042/bj3040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourey L., Samama J. P., Delarue M., Petitou M., Choay J., Moras D. Crystal structure of cleaved bovine antithrombin III at 3.2 A resolution. J Mol Biol. 1993 Jul 5;232(1):223–241. doi: 10.1006/jmbi.1993.1378. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Halvorson H. R., Björk I. Quantitative characterization of the thrombin-heparin interaction. Discrimination between specific and nonspecific binding models. J Biol Chem. 1991 Apr 5;266(10):6342–6352. [PubMed] [Google Scholar]
- Osterman D. G., Griffin G. L., Senior R. M., Kaiser E. T., Deuel T. F. The carboxyl-terminal tridecapeptide of platelet factor 4 is a potent chemotactic agent for monocytes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):130–135. doi: 10.1016/0006-291x(82)91679-5. [DOI] [PubMed] [Google Scholar]
- Perlin A. S., Mackie D. M., Dietrich C. P. Evidence for a (1 leads to 4)-linked 4-O-( -L-idopyranosyluronic acid 2-sulfate)-(2-deoxy-2-sulfoamino-D-glucopyranosyl 6-sulfate) sequence in heparin. Long-range H-H coupling in 4-deoxy-hex-4-enopyranosides. Carbohydr Res. 1971 Jun;18(2):185–194. doi: 10.1016/s0008-6215(00)80341-9. [DOI] [PubMed] [Google Scholar]
- Pomerantz M. W., Owen W. G. A catalytic role for heparin. Evidence for a ternary complex of heparin cofactor thrombin and heparin. Biochim Biophys Acta. 1978 Jul 21;535(1):66–77. doi: 10.1016/0005-2795(78)90033-8. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Sato Y., Abe M., Takaki R. Platelet factor 4 blocks the binding of basic fibroblast growth factor to the receptor and inhibits the spontaneous migration of vascular endothelial cells. Biochem Biophys Res Commun. 1990 Oct 30;172(2):595–600. doi: 10.1016/0006-291x(90)90715-y. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. J., Byers H. R., Scott C. F., Bauer S. I., Maione T. E. Growth inhibition of murine melanoma and human colon carcinoma by recombinant human platelet factor 4. J Natl Cancer Inst. 1990 May 16;82(10):848–853. doi: 10.1093/jnci/82.10.848. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- St Charles R., Walz D. A., Edwards B. F. The three-dimensional structure of bovine platelet factor 4 at 3.0-A resolution. J Biol Chem. 1989 Feb 5;264(4):2092–2099. [PubMed] [Google Scholar]
- Stuckey J. A., St Charles R., Edwards B. F. A model of the platelet factor 4 complex with heparin. Proteins. 1992 Oct;14(2):277–287. doi: 10.1002/prot.340140213. [DOI] [PubMed] [Google Scholar]
- Talpas C. J., Walz D. A., Lee L. 1H-NMR studies of bovine platelet factor 4: histidine assignments and interactions with heparin. Biochim Biophys Acta. 1991 Jun 24;1078(2):208–218. doi: 10.1016/0167-4838(91)99011-g. [DOI] [PubMed] [Google Scholar]
- Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992 May 25;267(15):10337–10341. [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993 Mar 12;72(5):779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- Wallis M. On the frequency of arginine in proteins and its implications for molecular evolution. Biochem Biophys Res Commun. 1974 Feb 4;56(3):711–716. doi: 10.1016/0006-291x(74)90663-9. [DOI] [PubMed] [Google Scholar]
- Witt D. P., Lander A. D. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol. 1994 May 1;4(5):394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Yang Y., Mayo K. H., Daly T. J., Barry J. K., La Rosa G. J. Subunit association and structural analysis of platelet basic protein and related proteins investigated by 1H NMR spectroscopy and circular dichroism. J Biol Chem. 1994 Aug 5;269(31):20110–20118. [PubMed] [Google Scholar]
- Zhang X., Chen L., Bancroft D. P., Lai C. K., Maione T. E. Crystal structure of recombinant human platelet factor 4. Biochemistry. 1994 Jul 12;33(27):8361–8366. doi: 10.1021/bi00193a025. [DOI] [PubMed] [Google Scholar]
- Zhang X., Chen L., Bancroft D. P., Lai C. K., Maione T. E. Crystal structure of recombinant human platelet factor 4. Biochemistry. 1994 Jul 12;33(27):8361–8366. doi: 10.1021/bi00193a025. [DOI] [PubMed] [Google Scholar]
- van Tilbeurgh H., Roussel A., Lalouel J. M., Cambillau C. Lipoprotein lipase. Molecular model based on the pancreatic lipase x-ray structure: consequences for heparin binding and catalysis. J Biol Chem. 1994 Feb 11;269(6):4626–4633. [PubMed] [Google Scholar]