Abstract
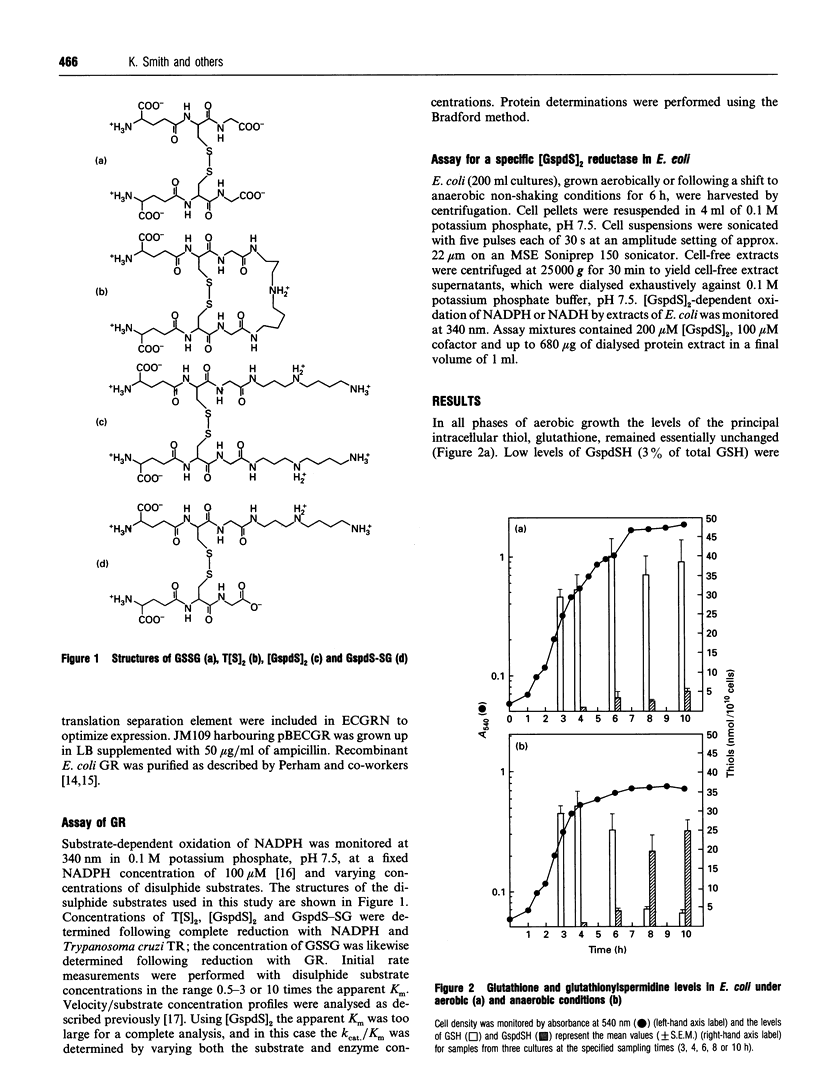
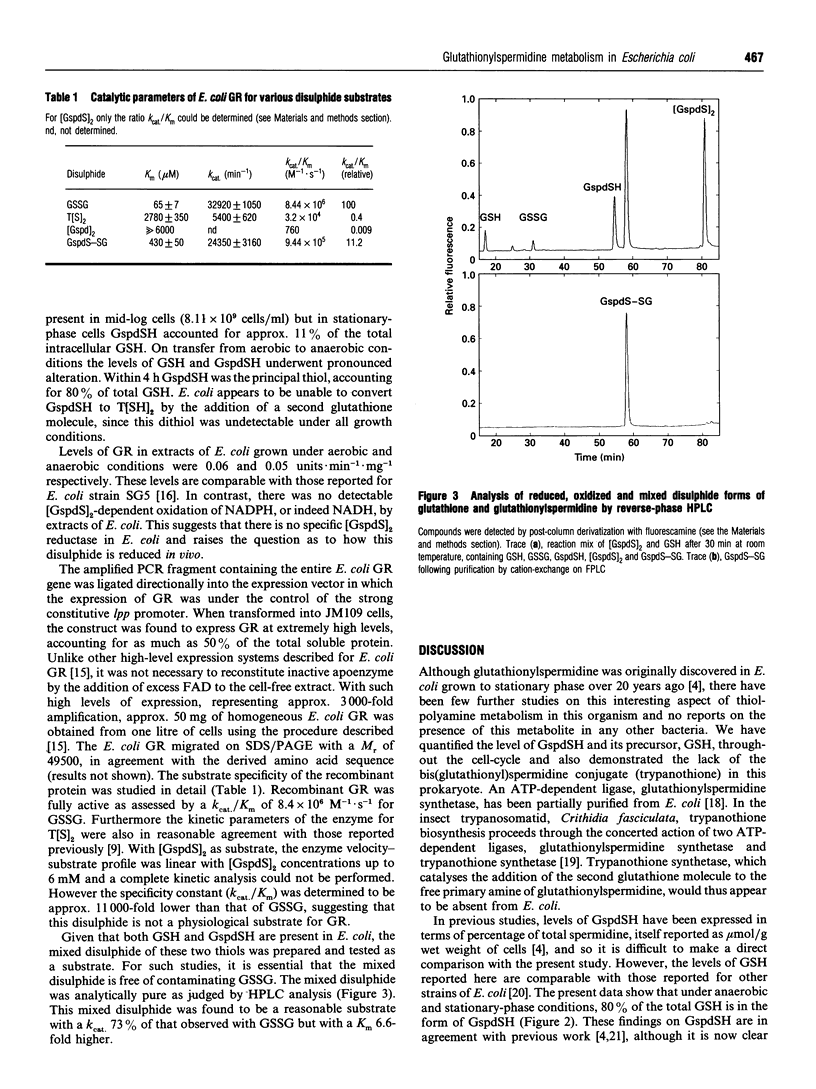
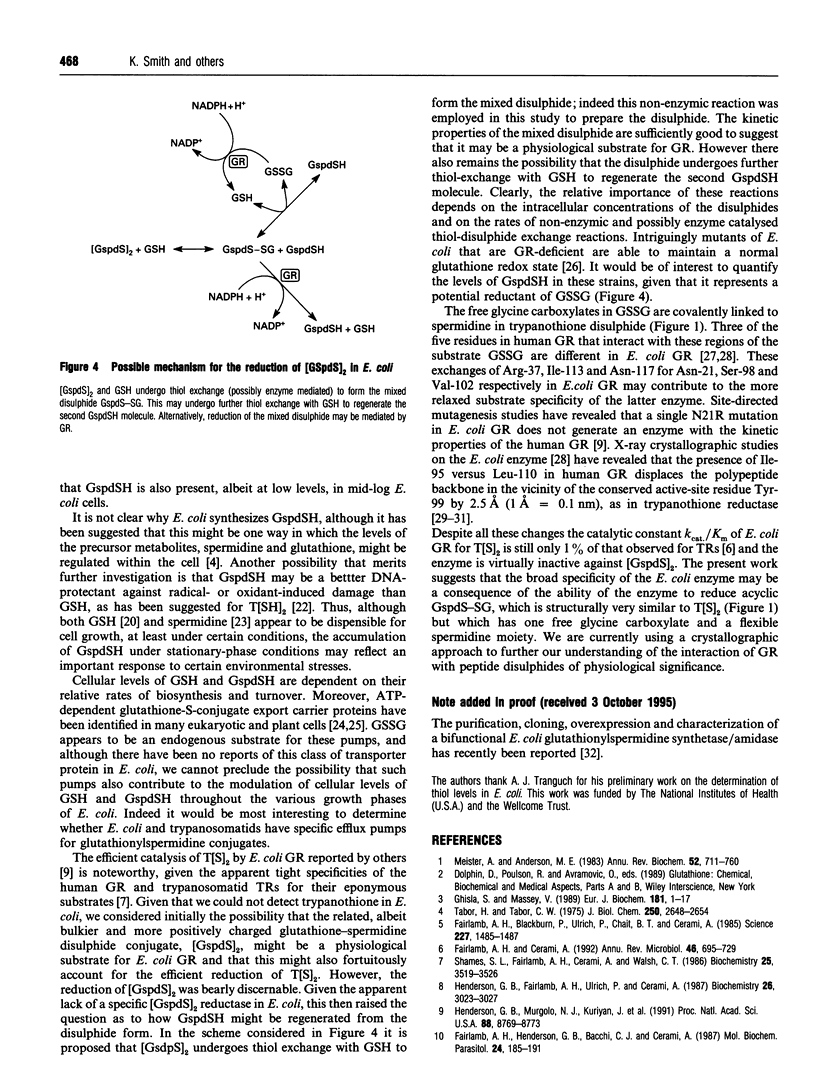
Intracellular levels of glutathione and glutathionylspermidine conjugates have been measured throughout the growth phases of Escherichia coli. Glutathionylspermidine was present in mid-log-phase cells, and under stationary and anaerobic growth conditions accounted for 80% of the total glutathione content. N1,N8-bis(glutathionyl)spermidine (trypanothione) was undetectable under all growth conditions. The catalytic constant kcat/Km of recombinant E. coli glutathione reductase for glutathionylspermidine disulphide was approx. 11,000-fold lower than that for glutathione disulphide. The much higher catalytic constant for the mixed disulphide of glutathione and glutathionylspermidine (11% that of GSSG), suggests a possible explanation for the low turnover of trypanothione disulphide by E. coli glutathione reductase, given the apparent lack of a specific glutathionylspermidine disulphide reductase in E. coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboagye-Kwarteng T., Smith K., Fairlamb A. H. Molecular characterization of the trypanothione reductase gene from Crithidia fasciculata and Trypanosoma brucei: comparison with other flavoprotein disulphide oxidoreductases with respect to substrate specificity and catalytic mechanism. Mol Microbiol. 1992 Nov;6(21):3089–3099. doi: 10.1111/j.1365-2958.1992.tb01766.x. [DOI] [PubMed] [Google Scholar]
- Awad S., Henderson G. B., Cerami A., Held K. D. Effects of trypanothione on the biological activity of irradiated transforming DNA. Int J Radiat Biol. 1992 Oct;62(4):401–407. doi: 10.1080/09553009214552281. [DOI] [PubMed] [Google Scholar]
- Bailey S., Smith K., Fairlamb A. H., Hunter W. N. Substrate interactions between trypanothione reductase and N1-glutathionylspermidine disulphide at 0.28-nm resolution. Eur J Biochem. 1993 Apr 1;213(1):67–75. doi: 10.1111/j.1432-1033.1993.tb17734.x. [DOI] [PubMed] [Google Scholar]
- Berry A., Scrutton N. S., Perham R. N. Switching kinetic mechanism and putative proton donor by directed mutagenesis of glutathione reductase. Biochemistry. 1989 Feb 7;28(3):1264–1269. doi: 10.1021/bi00429a047. [DOI] [PubMed] [Google Scholar]
- Bollinger J. M., Jr, Kwon D. S., Huisman G. W., Kolter R., Walsh C. T. Glutathionylspermidine metabolism in Escherichia coli. Purification, cloning, overproduction, and characterization of a bifunctional glutathionylspermidine synthetase/amidase. J Biol Chem. 1995 Jun 9;270(23):14031–14041. doi: 10.1074/jbc.270.23.14031. [DOI] [PubMed] [Google Scholar]
- Deonarain M. P., Berry A., Scrutton N. S., Perham R. N. Alternative proton donors/acceptors in the catalytic mechanism of the glutathione reductase of Escherichia coli: the role of histidine-439 and tyrosine-99. Biochemistry. 1989 Dec 12;28(25):9602–9607. doi: 10.1021/bi00451a008. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985 Mar 22;227(4693):1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Henderson G. B., Bacchi C. J., Cerami A. In vivo effects of difluoromethylornithine on trypanothione and polyamine levels in bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 1987 Jun;24(2):185–191. doi: 10.1016/0166-6851(87)90105-8. [DOI] [PubMed] [Google Scholar]
- Ghisla S., Massey V. Mechanisms of flavoprotein-catalyzed reactions. Eur J Biochem. 1989 Apr 15;181(1):1–17. doi: 10.1111/j.1432-1033.1989.tb14688.x. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986 Nov;168(2):1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer S., Perham R. N. Glutathione reductase from Escherichia coli: cloning and sequence analysis of the gene and relationship to other flavoprotein disulfide oxidoreductases. Biochemistry. 1986 May 6;25(9):2736–2742. doi: 10.1021/bi00357a069. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Fairlamb A. H., Ulrich P., Cerami A. Substrate specificity of the flavoprotein trypanothione disulfide reductase from Crithidia fasciculata. Biochemistry. 1987 Jun 2;26(11):3023–3027. doi: 10.1021/bi00385a011. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Murgolo N. J., Kuriyan J., Osapay K., Kominos D., Berry A., Scrutton N. S., Hinchliffe N. W., Perham R. N., Cerami A. Engineering the substrate specificity of glutathione reductase toward that of trypanothione reduction. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8769–8773. doi: 10.1073/pnas.88.19.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W. N., Bailey S., Habash J., Harrop S. J., Helliwell J. R., Aboagye-Kwarteng T., Smith K., Fairlamb A. H. Active site of trypanothione reductase. A target for rational drug design. J Mol Biol. 1992 Sep 5;227(1):322–333. doi: 10.1016/0022-2836(92)90701-k. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Kong X. P., Krishna T. S., Sweet R. M., Murgolo N. J., Field H., Cerami A., Henderson G. B. X-ray structure of trypanothione reductase from Crithidia fasciculata at 2.4-A resolution. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8764–8768. doi: 10.1073/pnas.88.19.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinnel T., Mechulam Y., Fayat G. Fast purification of a functional elongator tRNAmet expressed from a synthetic gene in vivo. Nucleic Acids Res. 1988 Aug 25;16(16):8095–8096. doi: 10.1093/nar/16.16.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mittl P. R., Schulz G. E. Structure of glutathione reductase from Escherichia coli at 1.86 A resolution: comparison with the enzyme from human erythrocytes. Protein Sci. 1994 May;3(5):799–809. doi: 10.1002/pro.5560030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Meijer C., Zaman G. J., Borst P., Scheper R. J., Mulder N. H., de Vries E. G., Jansen P. L. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton N. S., Berry A., Perham R. N. Purification and characterization of glutathione reductase encoded by a cloned and over-expressed gene in Escherichia coli. Biochem J. 1987 Aug 1;245(3):875–880. doi: 10.1042/bj2450875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames S. L., Fairlamb A. H., Cerami A., Walsh C. T. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulfide-containing flavoprotein reductases. Biochemistry. 1986 Jun 17;25(12):3519–3526. doi: 10.1021/bi00360a007. [DOI] [PubMed] [Google Scholar]
- Shim H., Fairlamb A. H. Levels of polyamines, glutathione and glutathione-spermidine conjugates during growth of the insect trypanosomatid Crithidia fasciculata. J Gen Microbiol. 1988 Mar;134(3):807–817. doi: 10.1099/00221287-134-3-807. [DOI] [PubMed] [Google Scholar]
- Smith K., Nadeau K., Bradley M., Walsh C., Fairlamb A. H. Purification of glutathionylspermidine and trypanothione synthetases from Crithidia fasciculata. Protein Sci. 1992 Jul;1(7):874–883. doi: 10.1002/pro.5560010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Glutathionylspermidine. Methods Enzymol. 1983;94:434–437. doi: 10.1016/s0076-6879(83)94078-8. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2648–2654. [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Glutathione reductase is not required for maintenance of reduced glutathione in Escherichia coli K-12. J Bacteriol. 1985 Apr;162(1):448–450. doi: 10.1128/jb.162.1.448-450.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Tabor C. W., Tabor H. Deletion mutations in the speED operon: spermidine is not essential for the growth of Escherichia coli. Gene. 1993 Apr 15;126(1):115–117. doi: 10.1016/0378-1119(93)90598-w. [DOI] [PubMed] [Google Scholar]
- el-Waer A., Douglas K. T., Smith K., Fairlamb A. H. Synthesis of N-benzyloxycarbonyl-L-cysteinylglycine 3-dimethylaminopropylamide disulfide: a cheap and convenient new assay for trypanothione reductase. Anal Biochem. 1991 Oct;198(1):212–216. doi: 10.1016/0003-2697(91)90531-w. [DOI] [PubMed] [Google Scholar]


