Abstract
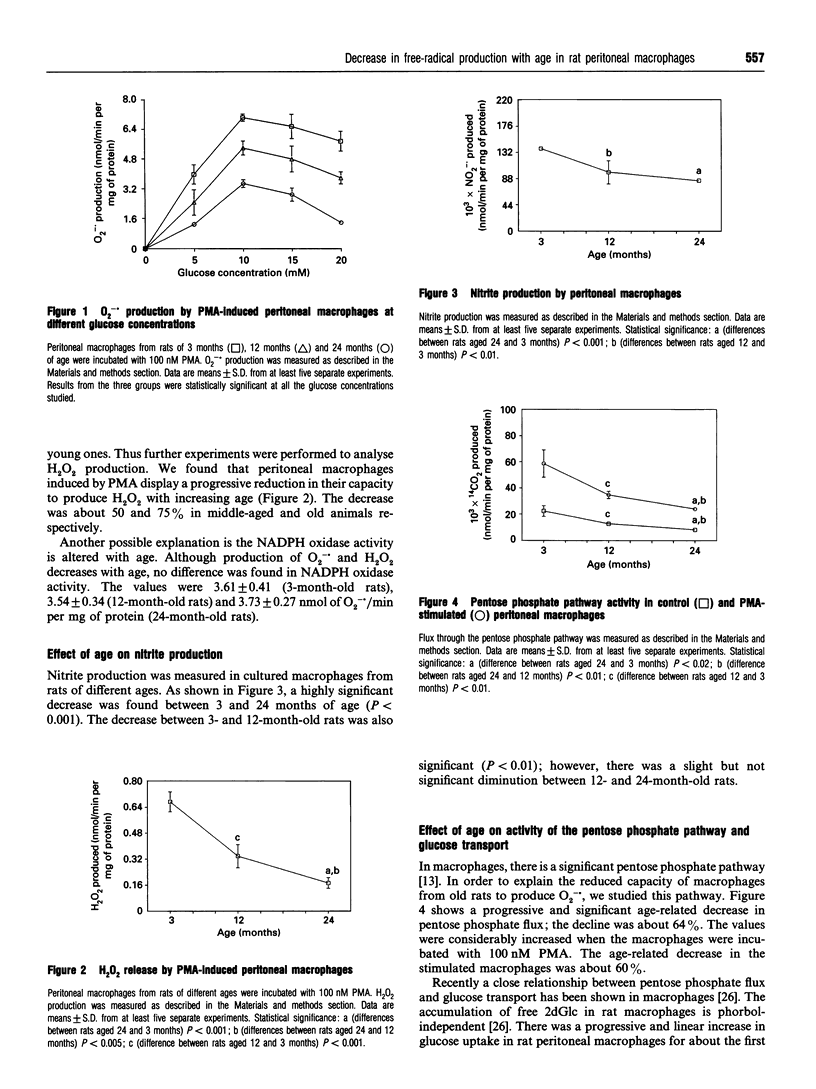
The respiratory-burst reaction has been studied in rat peritoneal macrophages of different ages (3, 12 and 24 months) using phorbol 12-myristate 13-acetate (PMA) to stimulate NADPH oxidase. Production of O2-. and H2O2 decreased with age (about 50 and 75% respectively); however, no difference in NADPH oxidase activity was found. NO. production was also reduced with age (40%). Furthermore, a progressive and significant decrease in the pentose phosphate flux was detected as a function of age in control and PMA-stimulated macrophages. The NADPH/NADP+ ratio decreased with age in control and PMA-stimulated macrophages. Glucose uptake was lower in middle-aged (12 months) and old (24 months) animals but no differences were found between these groups.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez E., Ruiz-Gutiérrez V., Santa María C., Machado A. Age-dependent modification of lipid composition and lipid structural order parameter of rat peritoneal macrophage membranes. Mech Ageing Dev. 1993 Oct 1;71(1-2):1–12. doi: 10.1016/0047-6374(93)90030-u. [DOI] [PubMed] [Google Scholar]
- Alvarez E., Santa María C., Machado A. Respiratory burst reaction changes with age in rat peritoneal macrophages. Biochim Biophys Acta. 1993 Nov 24;1179(3):247–252. doi: 10.1016/0167-4889(93)90079-5. [DOI] [PubMed] [Google Scholar]
- Antel J. P., Oger J. J., Dropcho E., Richman D. P., Kuo H. H., Arnason B. G. Reduced T-lymphocyte cell reactivity as a function of human aging. Cell Immunol. 1980 Aug 15;54(1):184–192. doi: 10.1016/0008-8749(80)90200-2. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr, Nathan D. G. Comparative study of the metabolic and bactericidal characteristics of severely glucose-6-phosphate dehydrogenase-deficient polymorphonuclear leukocytes and leukocytes from children with chronic granulomatous disease. J Reticuloendothel Soc. 1972 Aug;12(2):150–169. [PubMed] [Google Scholar]
- Borregaard N., Schwartz J. H., Tauber A. I. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984 Aug;74(2):455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Corradin S. B., Mauël J. Macrophage activation for intracellular killing as induced by a Ca2+ ionophore. Dependence on L-arginine-derived nitrogen oxidation products. Biochem J. 1992 Jun 1;284(Pt 2):387–392. doi: 10.1042/bj2840387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara M. D., Bedoya F., Sobrino F. Cyclosporin A inhibits phorbol ester-induced activation of superoxide production in resident mouse peritoneal macrophages. Biochem J. 1989 Nov 15;264(1):21–26. doi: 10.1042/bj2640021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara M. D., Sobrino F. Modulation of the inhibition of respiratory burst in mouse macrophages by cyclosporin A: effect of in vivo treatment, glucocorticoids and the state of activation of cells. Immunology. 1991 Jan;72(1):133–137. [PMC free article] [PubMed] [Google Scholar]
- Cooper M. R., DeChatelet L. R., McCall C. E., LaVia M. F., Spurr C. L., Baehner R. L. Complete deficiency of leukocyte glucose-6-phosphate dehydrogenase with defective bactericidal activity. J Clin Invest. 1972 Apr;51(4):769–778. doi: 10.1172/JCI106871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corraliza I. M., Campo M. L., Fuentes J. M., Campos-Portuguez S., Soler G. Parallel induction of nitric oxide and glucose-6-phosphate dehydrogenase in activated bone marrow derived macrophages. Biochem Biophys Res Commun. 1993 Oct 15;196(1):342–347. doi: 10.1006/bbrc.1993.2254. [DOI] [PubMed] [Google Scholar]
- Danon D., Kowatch M. A., Roth G. S. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L., Huber G. L. Hydroxyl radical formation in phagocytic cells of the rat. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jan;46(1):136–140. doi: 10.1152/jappl.1979.46.1.136. [DOI] [PubMed] [Google Scholar]
- Esposito A. L., Clark C. A., Poirier W. J. An assessment of the respiratory burst and bactericidal activity of alveolar macrophages from adult and senescent mice. J Leukoc Biol. 1988 May;43(5):445–454. doi: 10.1002/jlb.43.5.445. [DOI] [PubMed] [Google Scholar]
- Finger H., Heymer B., Wirsing von König C. H., Emmerling P. Macrophage function in senescence. Gerontology. 1982;28(4):223–232. doi: 10.1159/000212537. [DOI] [PubMed] [Google Scholar]
- Fink R. I., Huecksteadt T., Karaoghlanian Z. The effects of aging on glucose metabolism in adipocytes from Fischer rats. Endocrinology. 1986 Mar;118(3):1139–1147. doi: 10.1210/endo-118-3-1139. [DOI] [PubMed] [Google Scholar]
- Fink R. I., Kolterman O. G., Kao M., Olefsky J. M. The role of the glucose transport system in the postreceptor defect in insulin action associated with human aging. J Clin Endocrinol Metab. 1984 Apr;58(4):721–725. doi: 10.1210/jcem-58-4-721. [DOI] [PubMed] [Google Scholar]
- Fink R. I., Wallace P., Olefsky J. M. Effects of aging on glucose-mediated glucose disposal and glucose transport. J Clin Invest. 1986 Jun;77(6):2034–2041. doi: 10.1172/JCI112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., CLARK J. B., MCLEAN P. THE ESTIMATION OF THE OXIDIZED AND REDUCED FORMS OF THE NICOTINAMIDE NUCLEOTIDES. Biochem J. 1965 Apr;95:161–166. doi: 10.1042/bj0950161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R., Shamblin P. B., Craig C. P. Microbicidal activity and superoxide production by macrophages in aging rats. Allerg Immunol (Leipz) 1984;30(4):225–229. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Heyneman R. A., Vercauteren R. E. Activation of a NADPH oxidase from horse polymorphonuclear leukocytes in a cell-free system. J Leukoc Biol. 1984 Dec;36(6):751–759. doi: 10.1002/jlb.36.6.751. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kolb H., Burkart V. Nitric oxide production from macrophages is regulated by arachidonic acid metabolites. Biochem Biophys Res Commun. 1993 Nov 30;197(1):105–109. doi: 10.1006/bbrc.1993.2447. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Jaroslow B. N., Larrick J. W. Clearance of foreign red cells from the blood of aging mice. Mech Ageing Dev. 1973 Apr-May;2(1):23–32. doi: 10.1016/0047-6374(73)90005-5. [DOI] [PubMed] [Google Scholar]
- Lipschitz D. A., Udupa K. B., Boxer L. A. The role of calcium in the age-related decline of neutrophil function. Blood. 1988 Mar;71(3):659–665. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Melez K. A., Fries L. F., Bender B. S., Quinn T., Frank M. M. Decline in rates of clearance of IgG-sensitized erythrocytes with increasing age. Blood. 1988 Jun;71(6):1726–1730. [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Newsholme P., Gordon S., Newsholme E. A. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J. 1987 Mar 15;242(3):631–636. doi: 10.1042/bj2420631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson C. P., Hindson D. A. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes. 1989 Aug;38(8):1031–1035. doi: 10.2337/diab.38.8.1031. [DOI] [PubMed] [Google Scholar]
- Nohl H. Involvement of free radicals in ageing: a consequence or cause of senescence. Br Med Bull. 1993 Jul;49(3):653–667. doi: 10.1093/oxfordjournals.bmb.a072638. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Lin J. L., Tsukuda K., Katagiri H., Ishihara H., Inukai K., Yazaki Y. Expression of glucose transporter isoforms with aging. Gerontology. 1992;38 (Suppl 1):3–9. doi: 10.1159/000213355. [DOI] [PubMed] [Google Scholar]
- Oliver C. N. Inactivation of enzymes by activated human neutrophils. Curr Top Cell Regul. 1985;27:335–343. doi: 10.1016/b978-0-12-152827-0.50036-0. [DOI] [PubMed] [Google Scholar]
- Pellat-Deceunynck C., Wietzerbin J., Drapier J. C. Nicotinamide inhibits nitric oxide synthase mRNA induction in activated macrophages. Biochem J. 1994 Jan 1;297(Pt 1):53–58. doi: 10.1042/bj2970053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss M., Roos D. Differences in oxygen metabolism of phagocytosing monocytes and neutrophils. J Clin Invest. 1978 Feb;61(2):480–488. doi: 10.1172/JCI108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla E., Fabregat I., Santa María C., Machado A. The NADPH-producing pathways (pentose phosphate and malic enzyme) are regulated by the NADPH consumption in rat mammary gland. Biochem Int. 1987 May;14(5):957–962. [PubMed] [Google Scholar]
- Rist R. J., Jones G. E., Naftalin R. J. Synergistic activation of 2-deoxy-D-glucose uptake in rat and murine peritoneal macrophages by human macrophage colony-stimulating factor-stimulated coupling between transport and hexokinase activity and phorbol-dependent stimulation of pentose phosphate-shunt activity. Biochem J. 1990 Jan 1;265(1):243–249. doi: 10.1042/bj2650243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa L. F., De Almeida A. F., Safi D. A., Curi R. Metabolic and functional changes in lymphocytes and macrophages as induced by ageing. Physiol Behav. 1993 Apr;53(4):651–656. doi: 10.1016/0031-9384(93)90169-g. [DOI] [PubMed] [Google Scholar]
- Santa Maria C., Machado A. Changes in some hepatic enzyme activities related to phase II drug metabolism in male and female rats as a function of age. Mech Ageing Dev. 1988 Aug;44(2):115–125. doi: 10.1016/0047-6374(88)90084-x. [DOI] [PubMed] [Google Scholar]
- Saran M., Michel C., Bors W. Reaction of NO with O2-. implications for the action of endothelium-derived relaxing factor (EDRF). Free Radic Res Commun. 1990;10(4-5):221–226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- Strolin Benedetti M., Dostert P., Marrari P., Cini M. Effect of ageing on tissue levels of amino acids involved in the nitric oxide pathway in rat brain. J Neural Transm Gen Sect. 1993;94(1):21–30. doi: 10.1007/BF01244980. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F. FAD and GSH participate in macrophage synthesis of nitric oxide. Biochem Biophys Res Commun. 1990 Apr 30;168(2):558–565. doi: 10.1016/0006-291x(90)92357-6. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Enzymatic basis of macrophage activation. Kinetic analysis of superoxide production in lysates of resident and activated mouse peritoneal macrophages and granulocytes. J Biol Chem. 1984 Apr 10;259(7):4305–4312. [PubMed] [Google Scholar]


