Abstract
Human alpha 2-macroglobulin (alpha 2M) is a proteinase inhibitor and carrier of certain growth factors, including transforming growth factor beta 1 (TGF-beta 1). The constitutively synthesized homologue of human alpha 2M in the adult rat is alpha 1M. Rat alpha 2M is an acute-phase reactant, expressed at high levels in experimental trauma, pregnancy and in certain pathological conditions. The physiological role of rat alpha 2M is not known. In this investigation, we demonstrated that rat alpha 1M and rat alpha 2M bind TGF-beta 1. The equilibrium dissociation constants (KD) for the binding of TGF-beta 1 to the native forms of alpha 1M and alpha 2M were 257 and 109 nM respectively. alpha 1M underwent conformational change when it reacted with methylamine. The resulting product bound TGF-beta 1 with higher affinity (32 nM). Methylamine-treated rat alpha 2M did not undergo conformational change and did not bind TGF-beta 1 with increased affinity. Previous studies suggest that the native conformation may be the principal form responsible for the cytokine-carrier activity of alpha 2M in plasma and serum-supplemented cell culture medium. To confirm that native rat alpha 2M is a more efficient TGF-beta 1 carrier than native alpha 1M, fetal bovine heart endothelial cell (FBHE) proliferation assays were performed. TGF-beta 1 (5 pM) inhibited FBHE proliferation, and native alpha 2M (0.3 microM) counteracted this activity whereas alpha 1M (0.3 microM) had almost no effect. Rat alpha 2M underwent conformational change when it reacted with plasmin incorporating 1.1 mol of plasmin/mol. alpha 2M-plasmin bound TGF-beta 1; the KD (61 nM) was lower (P < 0.01) than that determined for the native alpha 2M-TGF-beta 1 interaction. These studies demonstrate that both rat alpha-macroglobulins are carriers of TGF-beta 1. The native form of rat alpha 2M probably has a predominant role, compared with native alpha 1M, as a TGF-beta 1 carrier in the plasma during the acute-phase response.
Full text
PDF


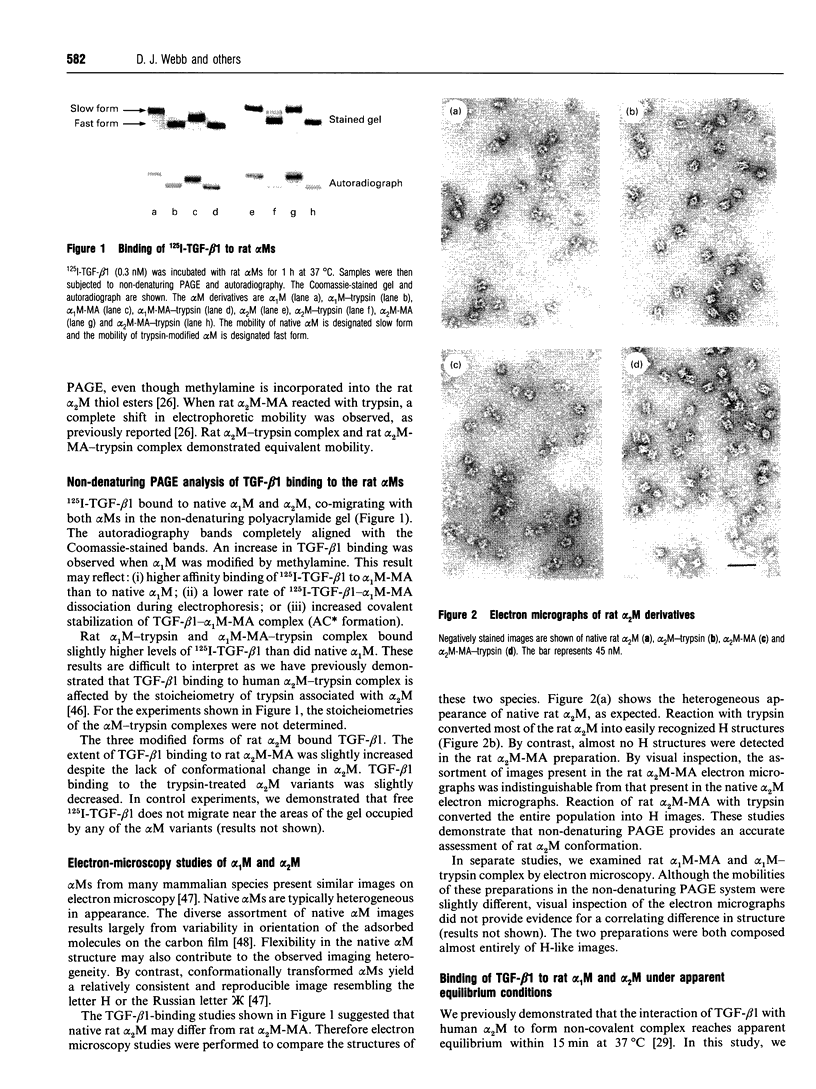
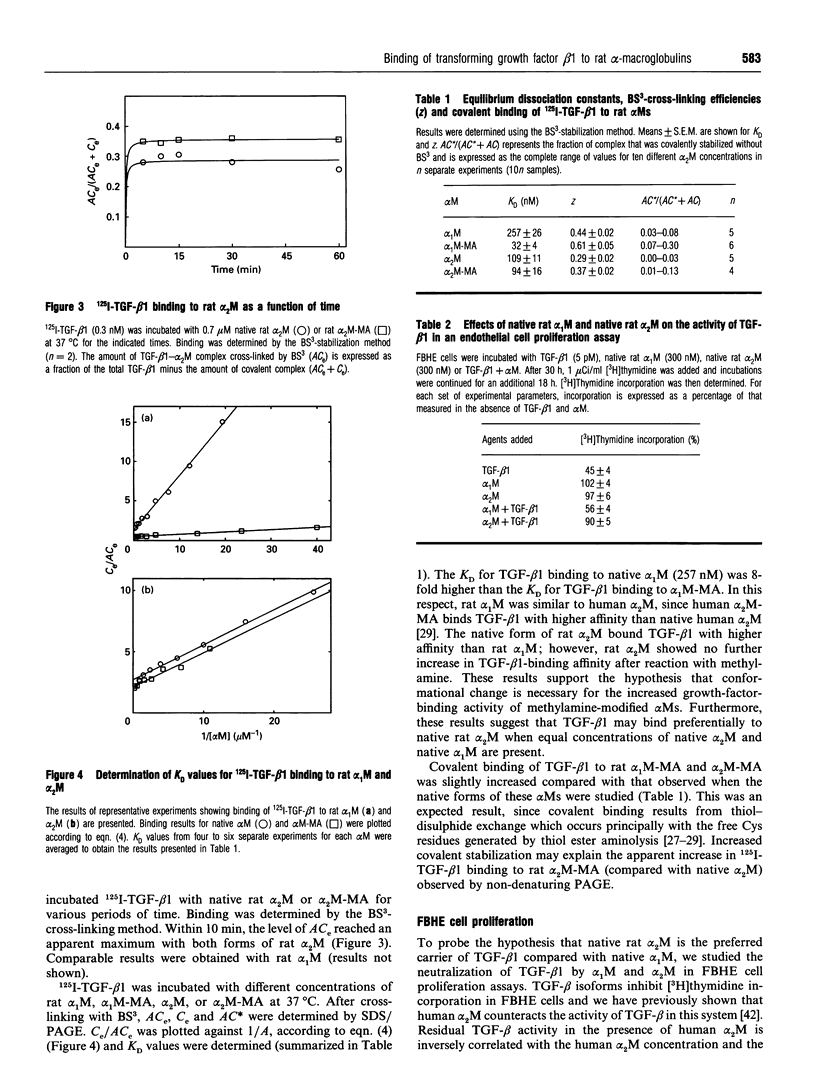
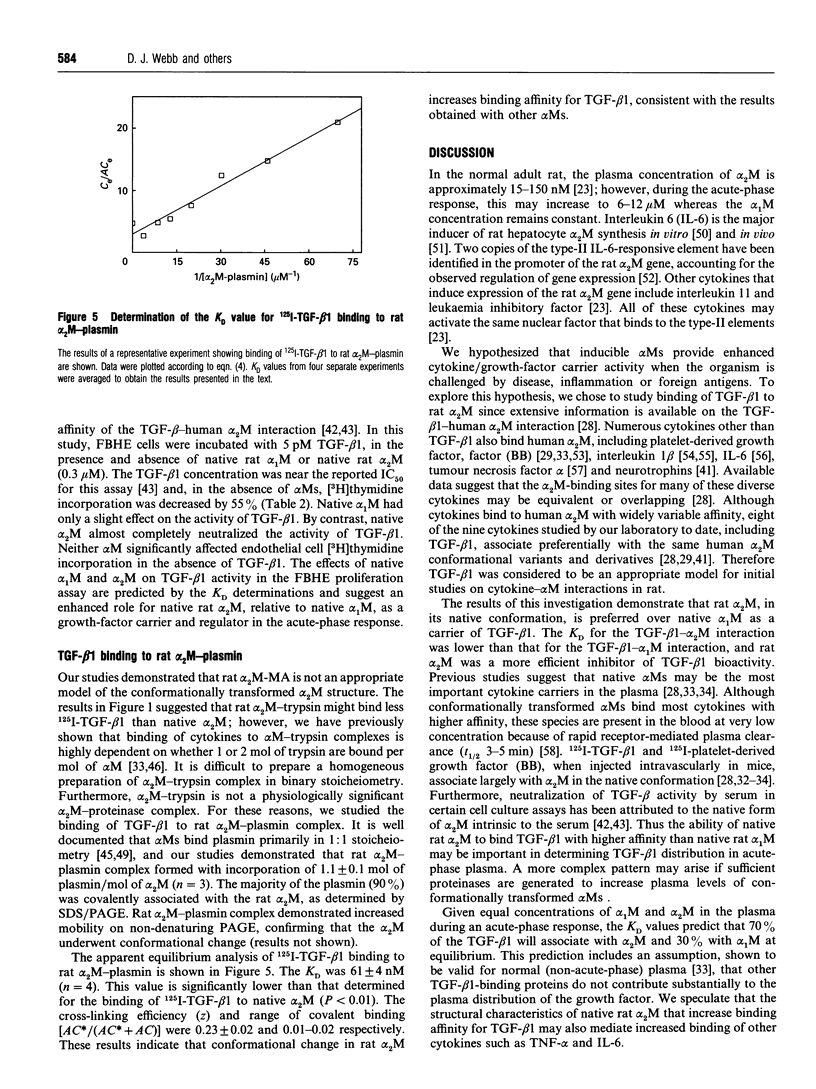


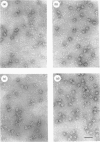
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Geiger T., Hirano T., Kishimoto T., Tran-Thi T. A., Decker K., Heinrich P. C. Regulation of synthesis and secretion of major rat acute-phase proteins by recombinant human interleukin-6 (BSF-2/IL-6) in hepatocyte primary cultures. Eur J Biochem. 1988 Apr 15;173(2):287–293. doi: 10.1111/j.1432-1033.1988.tb13997.x. [DOI] [PubMed] [Google Scholar]
- Anonick P. K., Gonias S. L. Soluble fibrin preparations inhibit the reaction of plasmin with alpha 2-macroglobulin. Comparison with alpha 2-antiplasmin and leupeptin. Biochem J. 1991 Apr 1;275(Pt 1):53–59. doi: 10.1042/bj2750053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcom J. D., Tiller S. E., Dickerson K., Cravens J. L., Argraves W. S., Strickland D. K. The human alpha 2-macroglobulin receptor: identification of a 420-kD cell surface glycoprotein specific for the activated conformation of alpha 2-macroglobulin. J Cell Biol. 1990 Apr;110(4):1041–1048. doi: 10.1083/jcb.110.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Fish W. W. Evidence for similar conformational changes in alpha 2-macroglobulin on reaction with primary amines or proteolytic enzymes. Biochem J. 1982 Nov 1;207(2):347–356. doi: 10.1042/bj2070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borth W. Alpha 2-macroglobulin. A multifunctional binding and targeting protein with possible roles in immunity and autoimmunity. Ann N Y Acad Sci. 1994 Sep 10;737:267–272. doi: 10.1111/j.1749-6632.1994.tb44317.x. [DOI] [PubMed] [Google Scholar]
- Borth W., Luger T. A. Identification of alpha 2-macroglobulin as a cytokine binding plasma protein. Binding of interleukin-1 beta to "F" alpha 2-macroglobulin. J Biol Chem. 1989 Apr 5;264(10):5818–5825. [PubMed] [Google Scholar]
- Borth W., Urbanski A., Prohaska R., Susanj M., Luger T. A. Binding of recombinant interleukin-1 beta to the third complement component and alpha 2-macroglobulin after activation of serum by immune complexes. Blood. 1990 Jun 15;75(12):2388–2395. [PubMed] [Google Scholar]
- Burmester J. K., Qian S. W., Roberts A. B., Huang A., Amatayakul-Chantler S., Suardet L., Odartchenko N., Madri J. A., Sporn M. B. Characterization of distinct functional domains of transforming growth factor beta. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8628–8632. doi: 10.1073/pnas.90.18.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Crookston K. P., Webb D. J., Lamarre J., Gonias S. L. Binding of platelet-derived growth factor-BB and transforming growth factor-beta 1 to alpha 2-macroglobulin in vitro and in vivo: comparison of receptor-recognized and non-recognized alpha 2-macroglobulin conformations. Biochem J. 1993 Jul 15;293(Pt 2):443–450. doi: 10.1042/bj2930443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookston K. P., Webb D. J., Wolf B. B., Gonias S. L. Classification of alpha 2-macroglobulin-cytokine interactions based on affinity of noncovalent association in solution under apparent equilibrium conditions. J Biol Chem. 1994 Jan 14;269(2):1533–1540. [PubMed] [Google Scholar]
- Delain E., Pochon F., Barray M., Van Leuven F. Ultrastructure of alpha 2-macroglobulins. Electron Microsc Rev. 1992;5(2):231–281. doi: 10.1016/0892-0354(92)90012-f. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Eccleston E. D., Howard J. B. Reaction of methylamine with human alpha 2-macroglobulin. Mechanism of inactivation. J Biol Chem. 1985 Aug 25;260(18):10169–10176. [PubMed] [Google Scholar]
- Geiger T., Andus T., Klapproth J., Hirano T., Kishimoto T., Heinrich P. C. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988 May;18(5):717–721. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Einarsson M., Pizzo S. V. Catabolic pathways for streptokinase, plasmin, and streptokinase activator complex in mice. In vivo reaction of plasminogen activator with alpha 2-macroglobulin. J Clin Invest. 1982 Aug;70(2):412–423. doi: 10.1172/JCI110631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S. L., LaMarre J., Crookston K. P., Webb D. J., Wolf B. B., Lopes M. B., Moses H. L., Hayes M. A. Alpha 2-macroglobulin and the alpha 2-macroglobulin receptor/LRP. A growth regulatory axis. Ann N Y Acad Sci. 1994 Sep 10;737:273–290. doi: 10.1111/j.1749-6632.1994.tb44318.x. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Conformation and protease binding activity of binary and ternary human alpha 2-macroglobulin-protease complexes. J Biol Chem. 1983 Dec 10;258(23):14682–14685. [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Reaction of human alpha 2-macroglobulin half-molecules with plasmin as a probe of protease binding site structure. Biochemistry. 1983 Oct 11;22(21):4933–4940. doi: 10.1021/bi00290a009. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Reynolds J. A., Pizzo S. V. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982 Aug 10;705(3):306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Gordon A. H. The alpha macroglobulins of rat serum. Biochem J. 1976 Dec 1;159(3):643–650. doi: 10.1042/bj1590643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. W., LaMarre J., Marshall L. B., Hayes M. A., Gonias S. L. Binding of transforming growth factor-beta 1 to methylamine-modified alpha 2-macroglobulin and to binary and ternary alpha 2-macroglobulin-proteinase complexes. Biochem J. 1992 Jan 15;281(Pt 2):569–575. doi: 10.1042/bj2810569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B. Reactive site in human alpha 2-macroglobulin: circumstantial evidence for a thiolester. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2235–2239. doi: 10.1073/pnas.78.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Specific covalent binding of platelet-derived growth factor to human plasma alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):342–346. doi: 10.1073/pnas.81.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. S., O'Grady P., Huang J. S. Human transforming growth factor beta.alpha 2-macroglobulin complex is a latent form of transforming growth factor beta. J Biol Chem. 1988 Jan 25;263(3):1535–1541. [PubMed] [Google Scholar]
- Krause E., Wegenka U., Möller C., Horn F., Heinrich P. C. Gene expression of the high molecular weight proteinase inhibitor alpha 2-macroglobulin. Biol Chem Hoppe Seyler. 1992 Jul;373(7):509–515. doi: 10.1515/bchm3.1992.373.2.509. [DOI] [PubMed] [Google Scholar]
- LaMarre J., Hayes M. A., Wollenberg G. K., Hussaini I., Hall S. W., Gonias S. L. An alpha 2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-beta 1 in mice. J Clin Invest. 1991 Jan;87(1):39–44. doi: 10.1172/JCI114998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larquet E., Boisset N., Pochon F., Lamy J. Architecture of native human alpha 2-macroglobulin studied by cryoelectron microscopy and three-dimensional reconstruction. J Struct Biol. 1994 Jul-Aug;113(1):87–98. doi: 10.1006/jsbi.1994.1034. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Reed D. L., Roberts R. C., Damato-McCabe D. Three high molecular weight protease inhibitors of rat plasma. Reactions with trypsin. J Biol Chem. 1987 Apr 5;262(10):4844–4853. [PubMed] [Google Scholar]
- Lonberg-Holm K., Reed D. L., Roberts R. C., Hebert R. R., Hillman M. C., Kutney R. M. Three high molecular weight protease inhibitors of rat plasma. Isolation, characterization, and acute phase changes. J Biol Chem. 1987 Jan 5;262(1):438–445. [PubMed] [Google Scholar]
- Matsuda T., Hirano T., Nagasawa S., Kishimoto T. Identification of alpha 2-macroglobulin as a carrier protein for IL-6. J Immunol. 1989 Jan 1;142(1):148–152. [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Purification of the rat hepatic alpha 2-macroglobulin receptor as an approximately 440-kDa single chain protein. J Biol Chem. 1989 Sep 15;264(26):15574–15577. [PubMed] [Google Scholar]
- O'Connor-McCourt M. D., Wakefield L. M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987 Oct 15;262(29):14090–14099. [PubMed] [Google Scholar]
- Okubo H., Miyanaga O., Nagano M., Ishibashi H., Kudo J., Ikuta T., Shibata K. Purification and immunological determination of alpha 2-macroglobulin in serum from injured rats. Biochim Biophys Acta. 1981 Apr 28;668(2):257–267. doi: 10.1016/0005-2795(81)90033-7. [DOI] [PubMed] [Google Scholar]
- Panrucker D. E., Lorscheider F. L. Isolation and purification of rat acute-phase alpha 2-macroglobulin. Biochim Biophys Acta. 1982 Jul 26;705(2):174–183. doi: 10.1016/0167-4838(82)90176-5. [DOI] [PubMed] [Google Scholar]
- Pochon F., Favaudon V., Tourbez-Perrin M., Bieth J. Localization of the two protease binding sites in human alpha 2-macroglobulin. J Biol Chem. 1981 Jan 25;256(2):547–550. [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Strickland D. K., Enghild J. J., Pizzo S. V. Evidence that the platinum-reactive methionyl residue of the alpha 2-macroglobulin receptor recognition site is not in the carboxyl-terminal receptor binding domain. J Biol Chem. 1988 May 15;263(14):6715–6721. [PubMed] [Google Scholar]
- Ruff E., Rizzino A. Preparation and binding of radioactively labeled porcine transforming growth factor type beta. Biochem Biophys Res Commun. 1986 Jul 31;138(2):714–719. doi: 10.1016/s0006-291x(86)80555-1. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Gliemann J., Van Leuven F. Domain structure of human alpha 2-macroglobulin. Characterization of a receptor-binding domain obtained by digestion with papain. FEBS Lett. 1986 Sep 1;205(1):20–24. doi: 10.1016/0014-5793(86)80857-2. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Sand O., Kristensen L., Fey G. H. The alpha-macroglobulin bait region. Sequence diversity and localization of cleavage sites for proteinases in five mammalian alpha-macroglobulins. J Biol Chem. 1989 Sep 25;264(27):15781–15789. [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., Argraves W. S. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990 Oct 15;265(29):17401–17404. [PubMed] [Google Scholar]
- Van Leuven F., Marynen P., Sottrup-Jensen L., Cassiman J. J., Van den Berghe H. The receptor-binding domain of human alpha 2-macroglobulin. Isolation after limited proteolysis with a bacterial proteinase. J Biol Chem. 1986 Aug 25;261(24):11369–11373. [PubMed] [Google Scholar]
- Webb D. J., Atkins T. L., Crookston K. P., Burmester J. K., Qian S. W., Gonias S. L. Transforming growth factor beta isoform 2-specific high affinity binding to native alpha 2-macroglobulin. Chimeras identify a sequence that determines affinity for native but not activated alpha 2-macroglobulin. J Biol Chem. 1994 Dec 2;269(48):30402–30406. [PubMed] [Google Scholar]
- Webb D. J., LaMarre J., Gonias S. L. Effect of human alpha-thrombin on the transforming growth factor-beta 1-binding activity of human alpha 2-macroglobulin. Semin Thromb Hemost. 1992;18(3):305–310. doi: 10.1055/s-2007-1002569. [DOI] [PubMed] [Google Scholar]
- Wolf B. B., Gonias S. L. Neurotrophin binding to human alpha 2-macroglobulin under apparent equilibrium conditions. Biochemistry. 1994 Sep 20;33(37):11270–11277. doi: 10.1021/bi00203a024. [DOI] [PubMed] [Google Scholar]
- Wolf B. B., Lopes M. B., VandenBerg S. R., Gonias S. L. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992 Jul;141(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- Wollenberg G. K., LaMarre J., Rosendal S., Gonias S. L., Hayes M. A. Binding of tumor necrosis factor alpha to activated forms of human plasma alpha 2 macroglobulin. Am J Pathol. 1991 Feb;138(2):265–272. [PMC free article] [PubMed] [Google Scholar]