Abstract
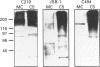
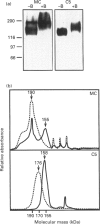
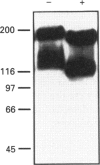
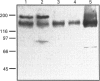
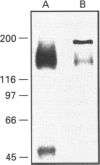
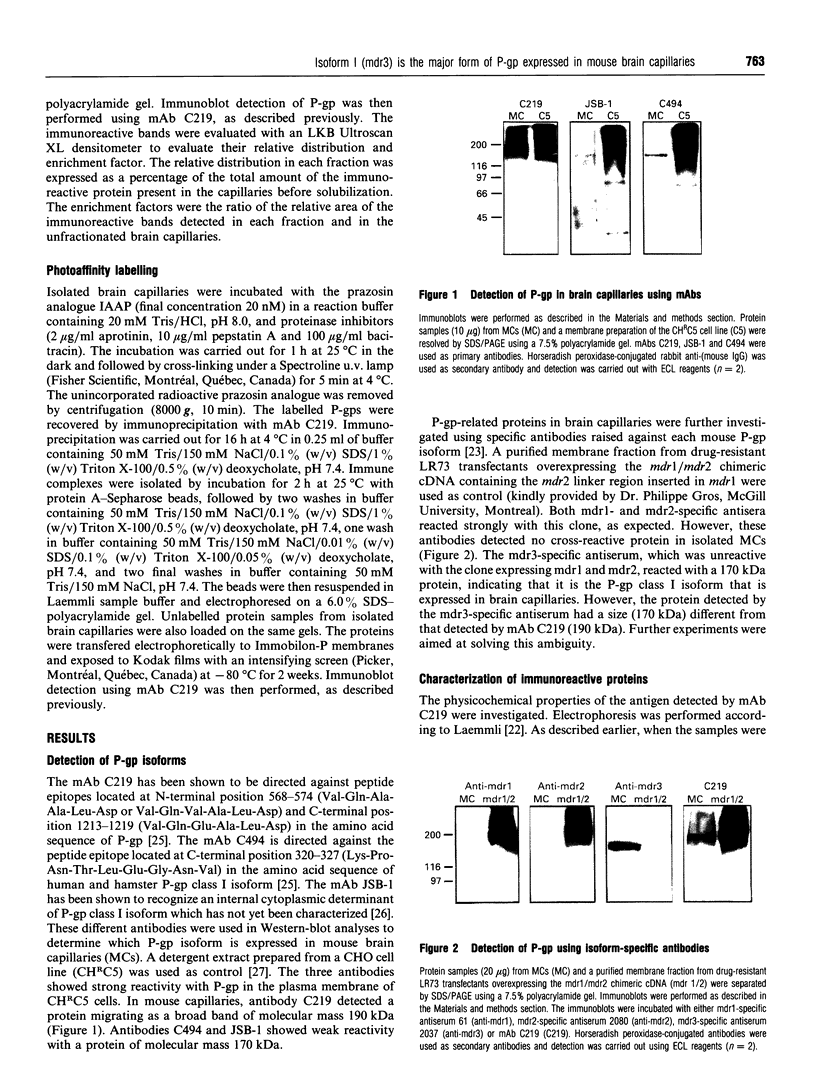
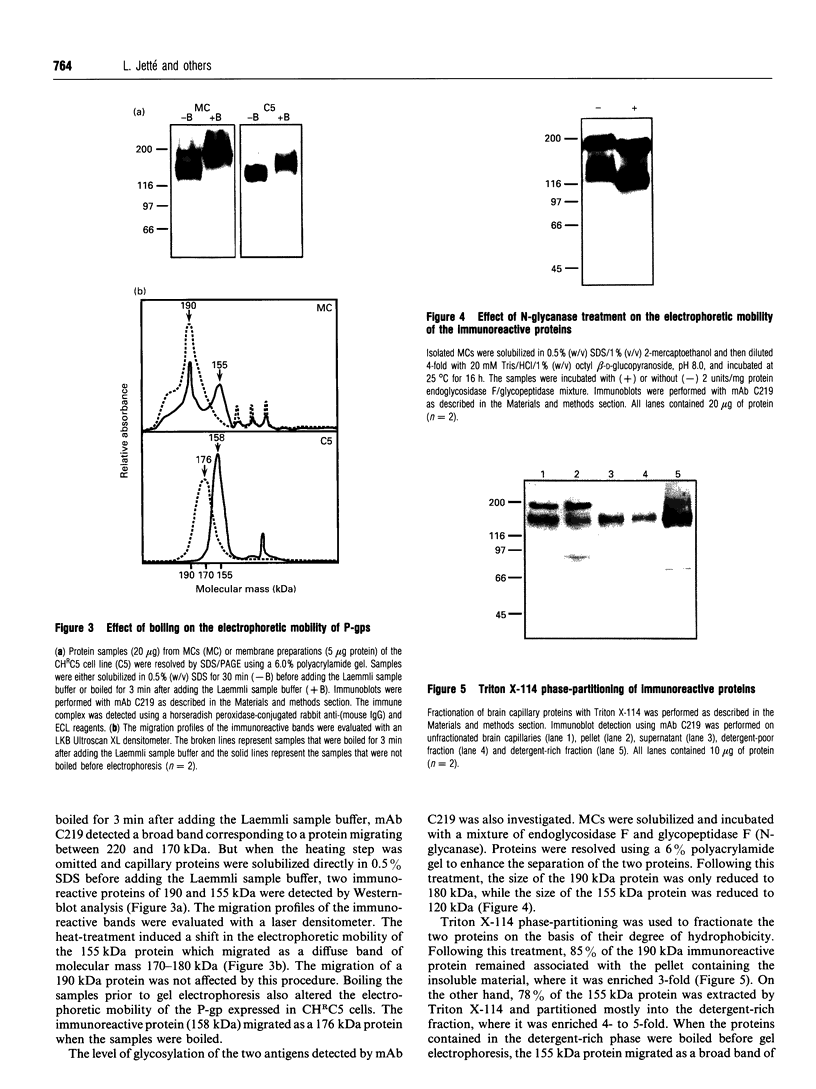
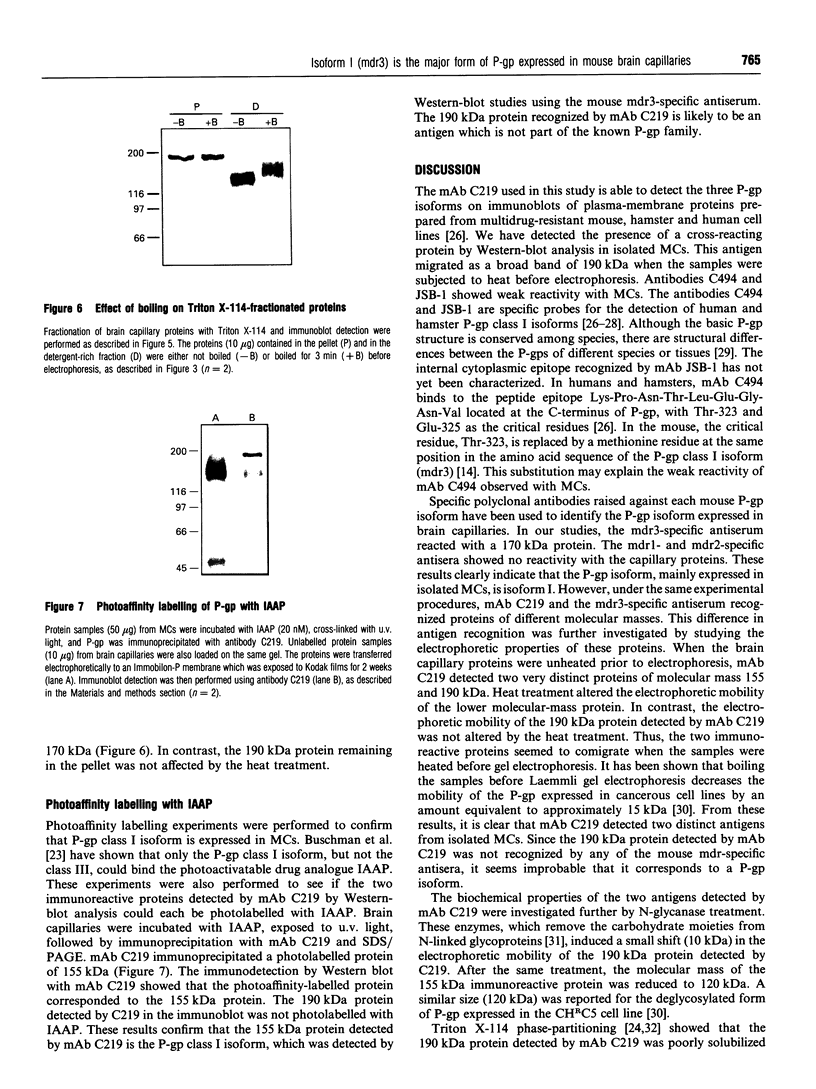
P-glycoprotein (P-gp) is expressed in various non-cancerous tissues such as the endothelial cells of the blood-brain barrier. We used several monoclonal antibodies (mAbs) and isoform-specific polyclonal antibodies to establish which P-gp isoforms are expressed in isolated mouse brain capillaries. P-gp class I isoform was detected in capillaries with a Western immunoblotting procedure using a specific antiserum. No immunoreactivity was observed with either class II- or class III-specific antisera. Immunoreactivity was observed with mAb C219. However, this antibody detected two distinct immunoreactive proteins (155 and 190 kDa) in the isolated brain capillaries. These two proteins comigrated as a broad band when the samples were submitted to heat prior to gel electrophoresis. The glycoprotein nature of these two antigens was evaluated by their sensitivity to N-glycanase treatment. Following this treatment, the size of the proteins was reduced from 190 and 155 kDa to 180 and 120 kDa, respectively. Triton X-114 phase-partitioning studies showed that the 190 kDa immunoreactive protein was poorly solubilized by Triton X-114, while the 155 kDa protein was partitioned in the detergent-rich phase. In labelling experiments, only the 155 kDa protein was photolabelled with [125I]iodoarylazidoprazosin. These results show that a 190 kDa protein detected by antibody C219 is an antigen unrelated to the three P-gp isoforms presently known. Cross-reactivity of C219 with an unrelated protein emphasizes the fact that more than one antibody should be used in the assessment of P-gp expression in cell lines and tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman E., Arceci R. J., Croop J. M., Che M., Arias I. M., Housman D. E., Gros P. mdr2 encodes P-glycoprotein expressed in the bile canalicular membrane as determined by isoform-specific antibodies. J Biol Chem. 1992 Sep 5;267(25):18093–18099. [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Deeley R. G. Multidrug resistance-associated protein: sequence correction. Science. 1993 May 14;260(5110):879–879. doi: 10.1126/science.8098549. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Casals D., Rittman-Grauer L., Biedler J. L., Melamed M. R., Bertino J. R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989 Jan;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., Housman D. E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989 Mar;9(3):1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire L., Tremblay L., Béliveau R. Purification and characterization of metabolically active capillaries of the blood-brain barrier. Biochem J. 1991 Jun 15;276(Pt 3):745–752. doi: 10.1042/bj2760745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Gros P. Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol. 1990 Apr;10(4):1652–1663. doi: 10.1128/mcb.10.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finstad C. L., Yin B. W., Gordon C. M., Federici M. G., Welt S., Lloyd K. O. Some monoclonal antibody reagents (C219 and JSB-1) to P-glycoprotein contain antibodies to blood group A carbohydrate determinants: a problem of quality control for immunohistochemical analysis. J Histochem Cytochem. 1991 Dec;39(12):1603–1610. doi: 10.1177/39.12.1682363. [DOI] [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Georges E., Bradley G., Gariepy J., Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. W., Betz A. L. Recent advances in understanding brain capillary function. Ann Neurol. 1983 Oct;14(4):389–395. doi: 10.1002/ana.410140402. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Greenberger L. M. Major photoaffinity drug labeling sites for iodoaryl azidoprazosin in P-glycoprotein are within, or immediately C-terminal to, transmembrane domains 6 and 12. J Biol Chem. 1993 May 25;268(15):11417–11425. [PubMed] [Google Scholar]
- Greenberger L. M., Williams S. S., Georges E., Ling V., Horwitz S. B. Electrophoretic analysis of P-glycoproteins produced by mouse J774.2 and Chinese hamster ovary multidrug-resistant cells. J Natl Cancer Inst. 1988 Jun 1;80(7):506–510. doi: 10.1093/jnci/80.7.506. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegmann E. J., Bauer H. C., Kerbel R. S. Expression and functional activity of P-glycoprotein in cultured cerebral capillary endothelial cells. Cancer Res. 1992 Dec 15;52(24):6969–6975. [PubMed] [Google Scholar]
- Henson J. W., Cordon-Cardo C., Posner J. B. P-glycoprotein expression in brain tumors. J Neurooncol. 1992 Sep;14(1):37–43. doi: 10.1007/BF00170943. [DOI] [PubMed] [Google Scholar]
- Jetté L., Têtu B., Béliveau R. High levels of P-glycoprotein detected in isolated brain capillaries. Biochim Biophys Acta. 1993 Aug 15;1150(2):147–154. doi: 10.1016/0005-2736(93)90083-c. [DOI] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Krishnamachary N., Center M. S. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993 Aug 15;53(16):3658–3661. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Lum B. L., Gosland M. P., Kaubisch S., Sikic B. I. Molecular targets in oncology: implications of the multidrug resistance gene. Pharmacotherapy. 1993 Mar-Apr;13(2):88–109. [PubMed] [Google Scholar]
- Nabors M. W., Griffin C. A., Zehnbauer B. A., Hruban R. H., Phillips P. C., Grossman S. A., Brem H., Colvin O. M. Multidrug resistance gene (MDR1) expression in human brain tumors. J Neurosurg. 1991 Dec;75(6):941–946. doi: 10.3171/jns.1991.75.6.0941. [DOI] [PubMed] [Google Scholar]
- Ng W. F., Sarangi F., Zastawny R. L., Veinot-Drebot L., Ling V. Identification of members of the P-glycoprotein multigene family. Mol Cell Biol. 1989 Mar;9(3):1224–1232. doi: 10.1128/mcb.9.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. Recent advances in blood-brain barrier transport. Annu Rev Pharmacol Toxicol. 1988;28:25–39. doi: 10.1146/annurev.pa.28.040188.000325. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Schinkel A. H., Roelofs E. M., Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991 May 15;51(10):2628–2635. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tatsuta T., Naito M., Oh-hara T., Sugawara I., Tsuruo T. Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem. 1992 Oct 5;267(28):20383–20391. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989 Feb;37(2):159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Terasaki T., Takabatake Y., Tenda Y., Tamai I., Yamashima T., Moritani S., Tsuruo T., Yamashita J. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 1992;51(18):1427–1437. doi: 10.1016/0024-3205(92)90537-y. [DOI] [PubMed] [Google Scholar]
- Vachon V., Pouliot J. F., Laprade R., Béliveau R. Fractionation of renal brush border membrane proteins with Triton X-114 phase partitioning. Biochem Cell Biol. 1991 Feb-Mar;69(2-3):206–211. doi: 10.1139/o91-031. [DOI] [PubMed] [Google Scholar]
- van der Valk P., van Kalken C. K., Ketelaars H., Broxterman H. J., Scheffer G., Kuiper C. M., Tsuruo T., Lankelma J., Meijer C. J., Pinedo H. M. Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann Oncol. 1990;1(1):56–64. [PubMed] [Google Scholar]