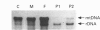Abstract
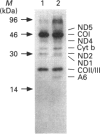
We have characterized cultured skin fibroblasts from two siblings affected with a fatal mitochondrial disease caused by a nuclear genetic defect. Mitochondrial respiratory-chain function was severely decreased in these cells. Southern-blot analysis showed that the fibroblasts had reduced levels of mitochondrial DNA (mtDNA). The mtDNA was unstable and was eliminated from the cultured cells over many generations, generating the rho0 genotype. As the mtDNA level decreased, the cells became more dependent upon pyruvate and uridine for growth. Nuclear-encoded subunits of respiratory-chain complexes were synthesized and imported into the mitochondria of the mtDNA-depleted cells, albeit at reduced levels compared with the controls. Mitochondrial protein synthesis directed by the residual mtDNA indicated that the mtDNA was expressed and that the defect specifically involves the replication or maintenance of mtDNA. This is a unique example of a respiratory-deficient human cell line exhibiting defective mtDNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Bodnar A. G., Cooper J. M., Holt I. J., Leonard J. V., Schapira A. H. Nuclear complementation restores mtDNA levels in cultured cells from a patient with mtDNA depletion. Am J Hum Genet. 1993 Sep;53(3):663–669. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Nuclear gadgets in mitochondrial DNA replication and transcription. Trends Biochem Sci. 1991 Mar;16(3):107–111. doi: 10.1016/0968-0004(91)90043-u. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Hyland K., Brand M., Leonard J. V. Mitochondrial phosphoenolpyruvate carboxykinase deficiency. Eur J Pediatr. 1986 Apr;145(1-2):46–50. doi: 10.1007/BF00441851. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Soderberg K., Landy F., Scheffler I. E. The selection of Chinese hamster cells deficient in oxidative energy metabolism. Somatic Cell Genet. 1976 Jul;2(4):331–344. doi: 10.1007/BF01538838. [DOI] [PubMed] [Google Scholar]
- Gibb G. M., Ragan C. I. Identification of the subunits of bovine NADH dehydrogenase which are encoded by the mitochondrial genome. Biochem J. 1990 Feb 1;265(3):903–906. doi: 10.1042/bj2650903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf A. L., Kelly J. L., Lehman I. R. Yeast RPO41 gene product is required for transcription and maintenance of the mitochondrial genome. Proc Natl Acad Sci U S A. 1986 May;83(10):3391–3394. doi: 10.1073/pnas.83.10.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines A. M., Cooper J. M., Morgan-Hughes J. A., Clark J. B., Schapira A. H. One-step immunoaffinity purification of complex I subunits from beef heart mitochondria. Protein Expr Purif. 1992 Jun;3(3):223–227. doi: 10.1016/1046-5928(92)90018-r. [DOI] [PubMed] [Google Scholar]
- Hare J. F., Ching E., Attardi G. Isolation, subunit composition, and site of synthesis of human cytochrome c oxidase. Biochemistry. 1980 May 13;19(10):2023–2030. doi: 10.1021/bi00551a003. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kikuchi A., Takemitsu M., Goto Y., Nonaka I. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10614–10618. doi: 10.1073/pnas.88.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Shimoizumi H., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. Respiration-deficient cells are caused by a single point mutation in the mitochondrial tRNA-Leu (UUR) gene in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS). Am J Hum Genet. 1991 Sep;49(3):590–599. [PMC free article] [PubMed] [Google Scholar]
- Krige D., Carroll M. T., Cooper J. M., Marsden C. D., Schapira A. H. Platelet mitochondrial function in Parkinson's disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol. 1992 Dec;32(6):782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonard J. V., Hyland K., Furukawa N., Clayton P. T. Mitochondrial phosphoenolpyruvate carboxykinase deficiency. Eur J Pediatr. 1991 Jan;150(3):198–199. doi: 10.1007/BF01963566. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Shanske S., Tritschler H. J., Aprille J. R., Andreetta F., Bonilla E., Schon E. A., DiMauro S. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991 Mar;48(3):492–501. [PMC free article] [PubMed] [Google Scholar]
- Myers A. M., Pape L. K., Tzagoloff A. Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 1985 Aug;4(8):2087–2092. doi: 10.1002/j.1460-2075.1985.tb03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongor S., Riedl Z. A latex agglutination test for lectin binding. Anal Biochem. 1983 Feb 15;129(1):51–56. doi: 10.1016/0003-2697(83)90050-7. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Ward J., Goodyer P., Baudet A. Respiratory chain defects in the mitochondria of cultured skin fibroblasts from three patients with lacticacidemia. J Clin Invest. 1986 May;77(5):1422–1427. doi: 10.1172/JCI112453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servidei S., Zeviani M., Manfredi G., Ricci E., Silvestri G., Bertini E., Gellera C., Di Mauro S., Di Donato S., Tonali P. Dominantly inherited mitochondrial myopathy with multiple deletions of mitochondrial DNA: clinical, morphologic, and biochemical studies. Neurology. 1991 Jul;41(7):1053–1059. doi: 10.1212/wnl.41.7.1053. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Gough A. C., Leigh P. N., Summers B. A., Harding A. E., Maraganore D. M., Sturman S. G., Schapira A. H., Williams A. C., Maranganore D. M. Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson's disease. Lancet. 1992 Jun 6;339(8806):1375–1377. doi: 10.1016/0140-6736(92)91196-f. [DOI] [PubMed] [Google Scholar]
- Sun N. C., Chang C. C., Chu E. H. Mutant hamster cells exhibiting a pleiotropic effect on carbohydrate metabolism. Proc Natl Acad Sci U S A. 1975 Feb;72(2):469–473. doi: 10.1073/pnas.72.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler H. J., Andreetta F., Moraes C. T., Bonilla E., Arnaudo E., Danon M. J., Glass S., Zelaya B. M., Vamos E., Telerman-Toppet N. Mitochondrial myopathy of childhood associated with depletion of mitochondrial DNA. Neurology. 1992 Jan;42(1):209–217. doi: 10.1212/wnl.42.1.209. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990 Sep;54(3):211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Myers A. M. Genetics of mitochondrial biogenesis. Annu Rev Biochem. 1986;55:249–285. doi: 10.1146/annurev.bi.55.070186.001341. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Welter C., Dooley S., Blin N. A rapid protocol for the purification of mitochondrial DNA suitable for studying restriction fragment length polymorphisms. Gene. 1989 Nov 15;83(1):169–172. doi: 10.1016/0378-1119(89)90415-0. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Bresolin N., Gellera C., Bordoni A., Pannacci M., Amati P., Moggio M., Servidei S., Scarlato G., DiDonato S. Nucleus-driven multiple large-scale deletions of the human mitochondrial genome: a new autosomal dominant disease. Am J Hum Genet. 1990 Dec;47(6):904–914. [PMC free article] [PubMed] [Google Scholar]
- Zeviani M., Servidei S., Gellera C., Bertini E., DiMauro S., DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989 May 25;339(6222):309–311. doi: 10.1038/339309a0. [DOI] [PubMed] [Google Scholar]