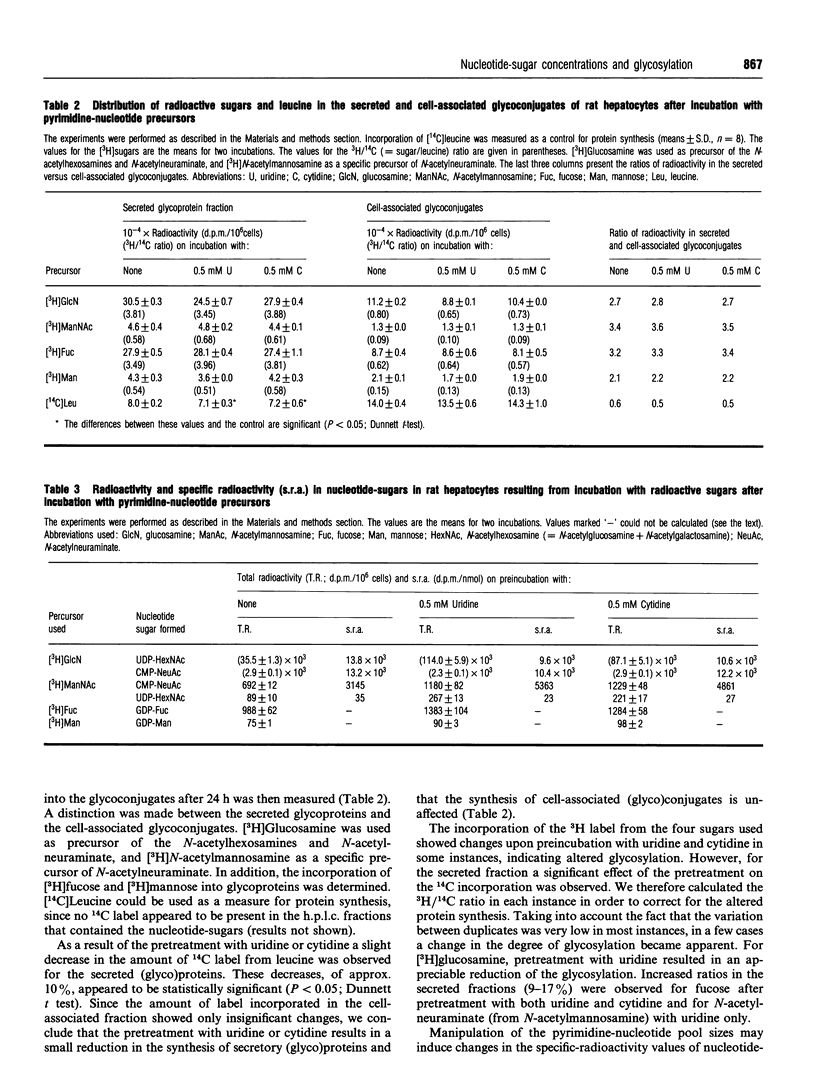
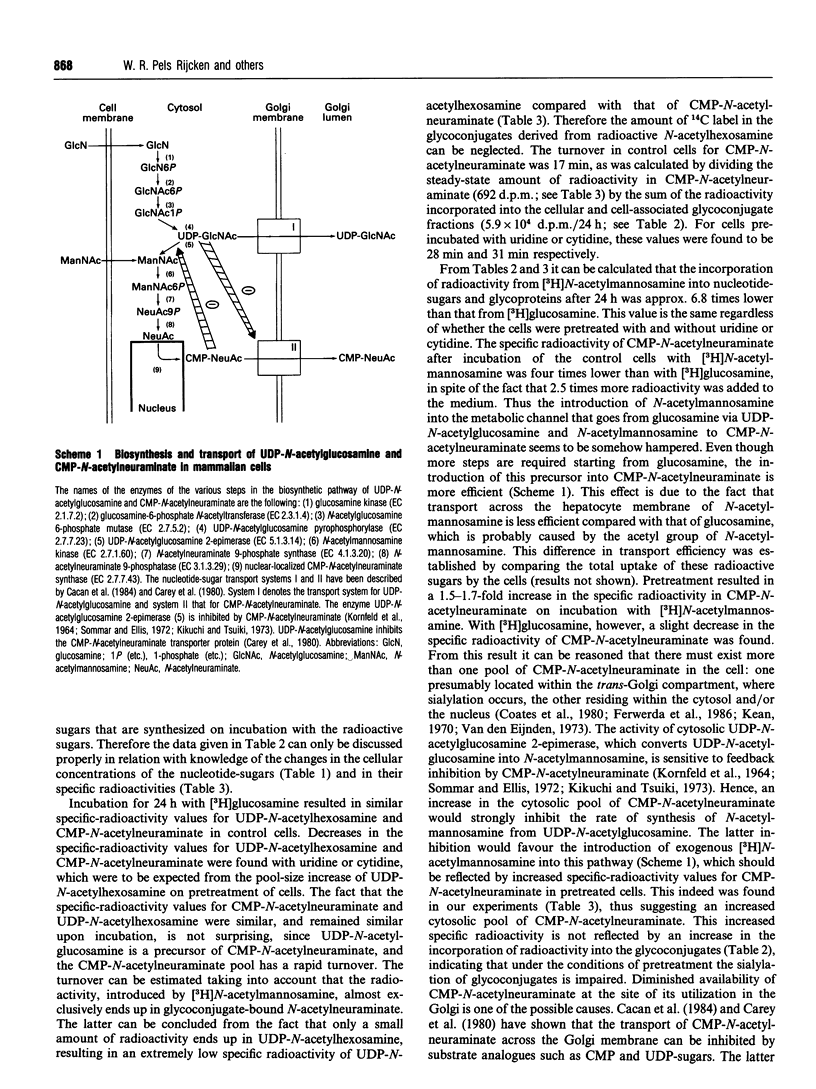
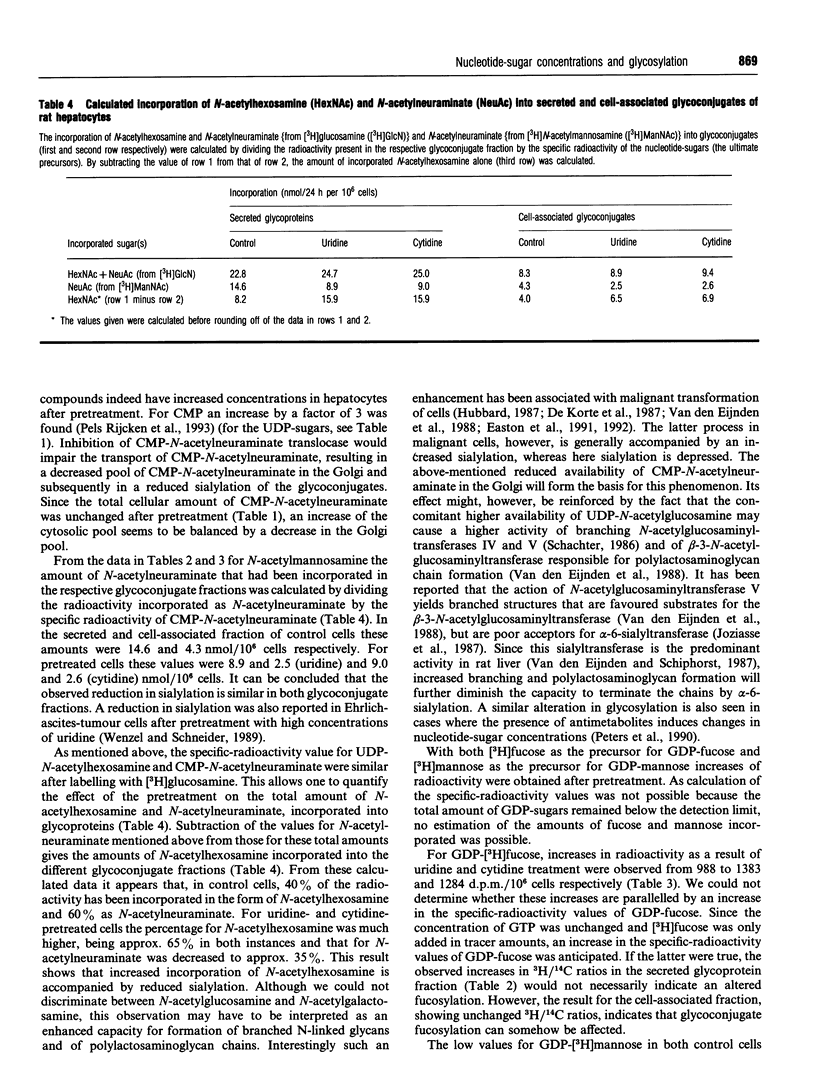
Abstract
Treatment of rat hepatocytes with 0.5 mM concentrations of uridine and cytidine results in increased cellular concentrations of UTP, UDP-sugars and CTP, whereas that of CMP-N-acetylneuraminate remained unchanged [Pels Rijcken, Overdijk, Van den Eijnden and Ferwerda (1993) Biochem. J. 293, 207-213]. The incorporation of radioactivity from 3H-labelled sugars into the cell-associated and secreted glycoconjugate fraction was influenced by these altered cellular concentrations of the nucleotides. For [3H]glucosamine, pretreatment with uridine resulted in a reduction of the glycosylation in both fractions. Increases in the secreted fractions were observed for fucose with both uridine and cytidine and for N-acetylglucosamine with uridine only. With [3H]N-acetylglucosamine, similar specific radioactivities for UDP-N-acetylhexosamine and CMP-N-acetylneuraminate were found, regardless of the pretreatment conditions. With [3H]N-acetylmannosamine, the specific radioactivity of CMP-N-acetylneuraminate showed an almost 2-fold increase on pretreatment. The latter increase did not result in an increased incorporation of radioactivity into the glycoconjugates. It was estimated that, in untreated cells, the ratio of radioactivity incorporated from [3H]glucosamine into glycoconjugate-bound N-acetylhexosamine and N-acetylneuraminate amounted to 2:3. In pretreated cells this ratio changed to approx. 2:1. Overall, the data show that pretreatment resulted in an increased incorporation of N-acetylhexosamine into cell-associated and secreted glycoconjugates, accompanied by a reduction in sialylation. It was concluded that an increased availability of UDP-N-acetylhexosamine caused the increased incorporation of N-acetylhexosamine. The elevated cytosolic level of UDP-N-acetylhexosamine (and of compounds like CMP) is suggested to impair the transport of CMP-acetylneuraminate to the Golgi, resulting in reduced sialylation. This study demonstrates that protein glycosylation can be regulated at the level of the availability of the various nucleotide-sugars in the Golgi lumen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Bulet P., Hoflack B., Porchet M., Verbert A. Study of the conversion of GDP-mannose into GDP-fucose in Nereids: a biochemical marker of oocyte maturation. Eur J Biochem. 1984 Oct 15;144(2):255–259. doi: 10.1111/j.1432-1033.1984.tb08458.x. [DOI] [PubMed] [Google Scholar]
- Cacan R., Cecchelli R., Hoflack B., Verbert A. Intralumenal pool and transport of CMP-N-acetylneuraminic acid, GDP-fucose and UDP-galactose. Study with plasma-membrane-permeabilized mouse thymocytes. Biochem J. 1984 Nov 15;224(1):277–284. doi: 10.1042/bj2240277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D. J., Sommers L. W., Hirschberg C. B. CMP-N-acetylneuraminic acid: isolation from and penetration into mouse liver microsomes. Cell. 1980 Mar;19(3):597–605. doi: 10.1016/s0092-8674(80)80036-5. [DOI] [PubMed] [Google Scholar]
- Coates S. W., Gurney T., Jr, Sommers L. W., Yeh M., Hirschberg C. B. Subcellular localization of sugar nucleotide synthetases. J Biol Chem. 1980 Oct 10;255(19):9225–9229. [PubMed] [Google Scholar]
- Easton E. W., Blokland I., Geldof A. A., Rao B. R., van den Eijnden D. H. The metastatic potential of rat prostate tumor variant R3327-MatLyLu is correlated with an increased activity of N-acetylglucosaminyl transferase III and V. FEBS Lett. 1992 Aug 10;308(1):46–49. doi: 10.1016/0014-5793(92)81047-p. [DOI] [PubMed] [Google Scholar]
- Easton E. W., Bolscher J. G., van den Eijnden D. H. Enzymatic amplification involving glycosyltransferases forms the basis for the increased size of asparagine-linked glycans at the surface of NIH 3T3 cells expressing the N-ras proto-oncogene. J Biol Chem. 1991 Nov 15;266(32):21674–21680. [PubMed] [Google Scholar]
- GINSBURG V. Studies on the biosynthesis of guanosine diphosphate L-fucose. J Biol Chem. 1961 Sep;236:2389–2393. [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C. Differential effects of oncogenic transformation on N-linked oligosaccharide processing at individual glycosylation sites of viral glycoproteins. J Biol Chem. 1987 Dec 5;262(34):16403–16411. [PubMed] [Google Scholar]
- Joziasse D. H., Schiphorst W. E., Van den Eijnden D. H., Van Kuik J. A., Van Halbeek H., Vliegenthart J. F. Branch specificity of bovine colostrum CMP-sialic acid: Gal beta 1----4GlcNAc-R alpha 2----6-sialyltransferase. Sialylation of bi-, tri-, and tetraantennary oligosaccharides and glycopeptides of the N-acetyllactosamine type. J Biol Chem. 1987 Feb 15;262(5):2025–2033. [PubMed] [Google Scholar]
- KORNFELD S., KORNFELD R., NEUFELD E. F., O'BRIEN P. J. THE FEEDBACK CONTROL OF SUGAR NUCLEOTIDE BIOSYNTHESIS IN LIVER. Proc Natl Acad Sci U S A. 1964 Aug;52:371–379. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E. L. Nuclear cytidine 5'-monophosphosialic acid synthetase. J Biol Chem. 1970 May 10;245(9):2301–2308. [PubMed] [Google Scholar]
- Kikuchi K., Tsuiki S. Purification and properties of UDP-N-acetylglucosamine 2'-epimerase from rat liver. Biochim Biophys Acta. 1973 Nov 15;327(1):193–206. doi: 10.1016/0005-2744(73)90117-4. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to the Golgi. A rate-limiting step in protein maturation and secretion. J Biol Chem. 1988 Feb 15;263(5):2107–2110. [PubMed] [Google Scholar]
- Martin A., Ruggiero-Lopez D., Broquet P., Richard M., Louisot P. High-performance liquid chromatographic study of GDP-mannose and GDP-fucose metabolism. J Chromatogr. 1989 Dec 29;497:319–325. doi: 10.1016/0378-4347(89)80036-2. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pels Rijcken W. R., Hooghwinkel G. J., Ferwerda W. Pyrimidine metabolism and sugar nucleotide synthesis in rat liver. Biochem J. 1990 Mar 15;266(3):777–783. doi: 10.1042/bj2660777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pels Rijcken W. R., Overdijk B., van den Eijnden D. H., Ferwerda W. Pyrimidine nucleotide metabolism in rat hepatocytes: evidence for compartmentation of nucleotide pools. Biochem J. 1993 Jul 1;293(Pt 1):207–213. doi: 10.1042/bj2930207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. J., Pinedo H. M., Ferwerda W., de Graaf T. W., van Dijk W. Do antimetabolites interfere with the glycosylation of cellular glycoconjugates? Eur J Cancer. 1990 Apr;26(4):516–523. doi: 10.1016/0277-5379(90)90029-s. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Movement of proteins through the Golgi stack: a molecular dissection of vesicular transport. FASEB J. 1990 Mar;4(5):1460–1468. doi: 10.1096/fasebj.4.5.2407590. [DOI] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986 Mar;64(3):163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Sommar K. M., Ellis D. B. Uridine diphosphate N-acetyl-D-glucosamine-2-epimerase from rat liver. I. Catalytic and regulatory properties. Biochim Biophys Acta. 1972 May 12;268(2):581–589. doi: 10.1016/0005-2744(72)90355-5. [DOI] [PubMed] [Google Scholar]
- Van den Eijnden D. H., Schiphorst W. E. Detection of beta-galactosyl(1 leads to 4)N-acetylglucosaminide alpha(2 leads to 3)-sialyltransferase activity in fetal calf liver and other tissues. J Biol Chem. 1981 Apr 10;256(7):3159–3162. [PubMed] [Google Scholar]
- Verbert A., Cacan R., Cecchelli R. Membrane transport of sugar donors to the glycosylation sites. Biochimie. 1987 Feb;69(2):91–99. doi: 10.1016/0300-9084(87)90240-9. [DOI] [PubMed] [Google Scholar]
- Wenzel A., Schneider F. Sialic acid, galactose and fucose on the surface of Ehrlich ascites tumor cells grown in glucose-free medium in the presence of uridine. Biol Chem Hoppe Seyler. 1989 Mar;370(3):205–209. doi: 10.1515/bchm3.1989.370.1.205. [DOI] [PubMed] [Google Scholar]
- de Korte D., Haverkort W. A., de Boer M., van Gennip A. H., Roos D. Imbalance in the nucleotide pools of myeloid leukemia cells and HL-60 cells: correlation with cell-cycle phase, proliferation, differentiation, and transformation. Cancer Res. 1987 Apr 1;47(7):1841–1847. [PubMed] [Google Scholar]
- van den Eijnden D. H., Koenderman A. H., Schiphorst W. E. Biosynthesis of blood group i-active polylactosaminoglycans. Partial purification and properties of an UDP-GlcNAc:N-acetyllactosaminide beta 1----3-N-acetylglucosaminyltransferase from Novikoff tumor cell ascites fluid. J Biol Chem. 1988 Sep 5;263(25):12461–12471. [PubMed] [Google Scholar]
- van den Eijnden D. H. The subcellular localization of cytidine 5'-monophospho-N-acetylneuraminic acid synthetase in calf brain. J Neurochem. 1973 Oct;21(4):949–958. doi: 10.1111/j.1471-4159.1973.tb07539.x. [DOI] [PubMed] [Google Scholar]


