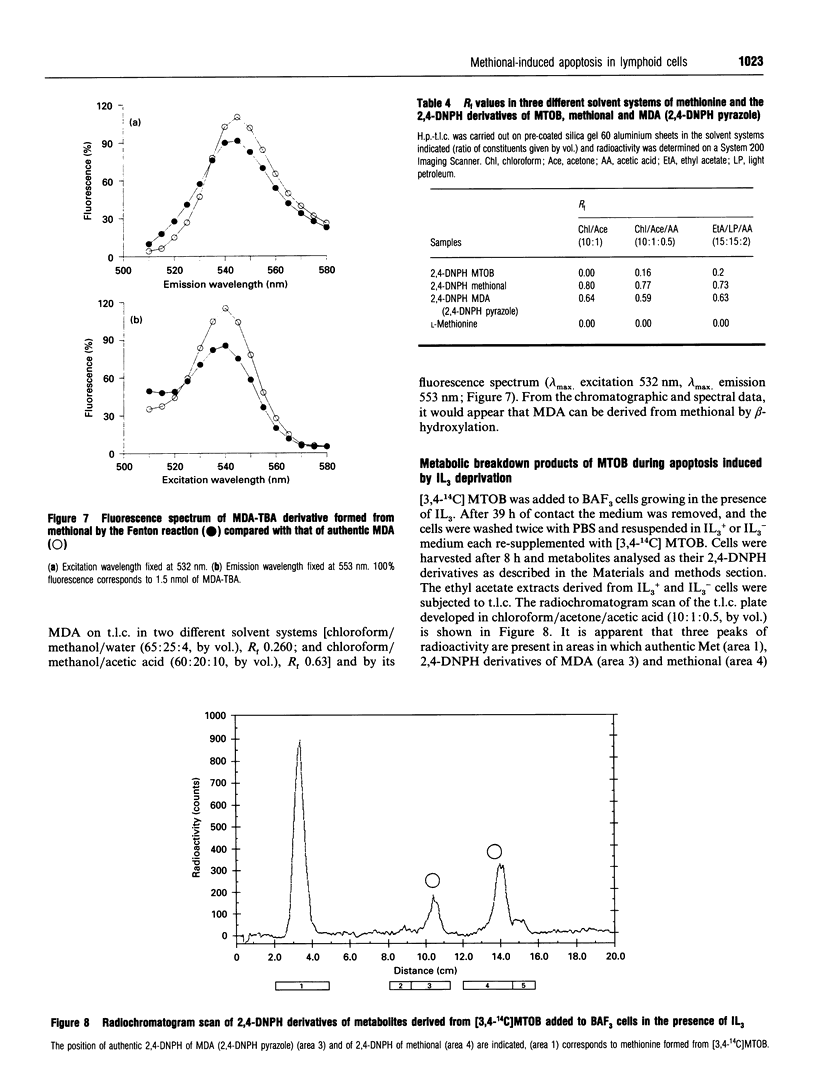
Abstract
4-Methylthio-2-oxobutanoic acid is the direct precursor of methional, which is a potent inducer of apoptosis in a BAF3 murine lymphoid cell line which is interleukin-3 (IL3)-dependent. Cultures treated for 8 h with methional in the presence of IL3 show extensive DNA double-strand breaks on flow cytometric analysis, increases in DNA fragmentation as measured by the amount of non-sedimentable DNA present in the 30,000 g supernatant of cell lysates and the typical laddering pattern of multiples of 180 bp seen upon agarose gel electrophoresis. No such features of apoptosis were found in cells treated with 4-methylthio 2-oxobutanoic acid or propanal, suggesting that the simultaneous presence of the methylthio group on the propanal moiety is essential for apoptosis to take place. Methional is further metabolized in cells by two reactions: oxidation via aldehyde dehydrogenase to (methylthio)propionic acid or beta-hydroxylation to malondialdehyde. The formation of malondialdehyde from methional in vitro by chemical hydroxylation under the conditions of the Fenton reaction provides a mechanism for the beta-hydroxylation which takes place in vivo. During apoptosis induced by IL3 deprivation, the ratio of 2,4-DNPH MDA to 2,4-DNPH methional is 0.94 in cells in IL3- medium compared with 0.54 in cells in IL3+ medium. These results support a role of cellular methional and malondialdehyde in apoptosis.
Full text
PDF


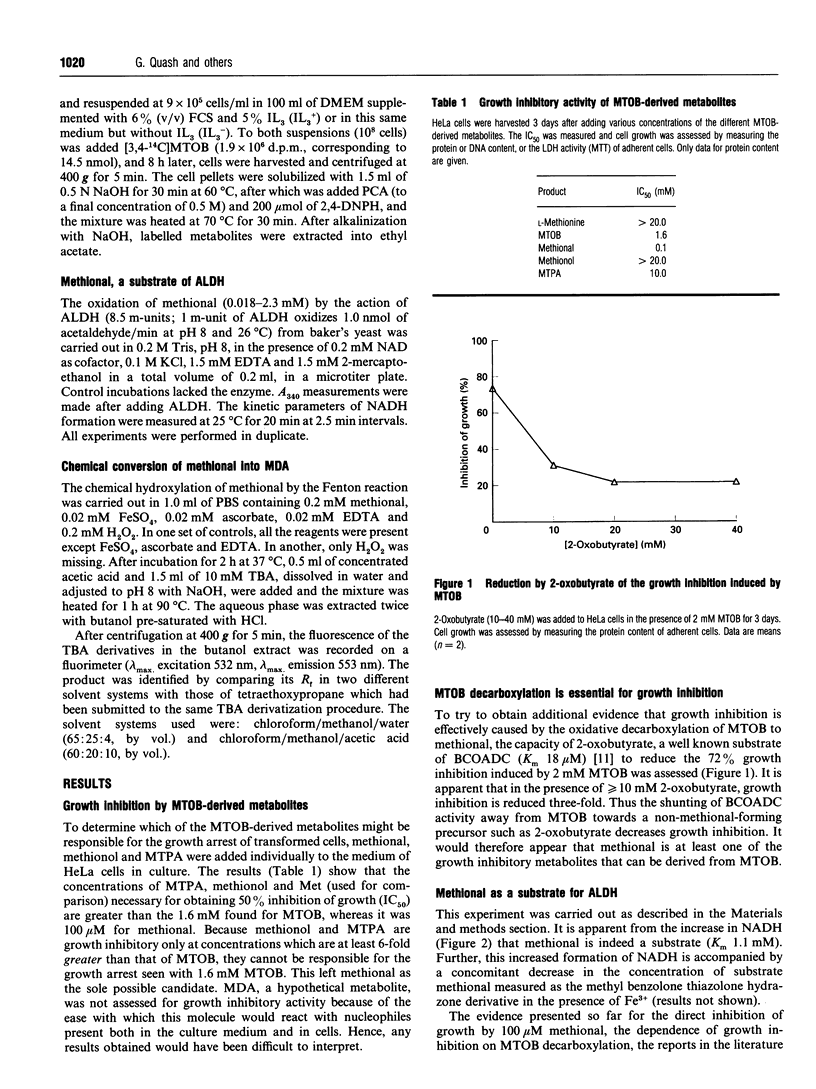
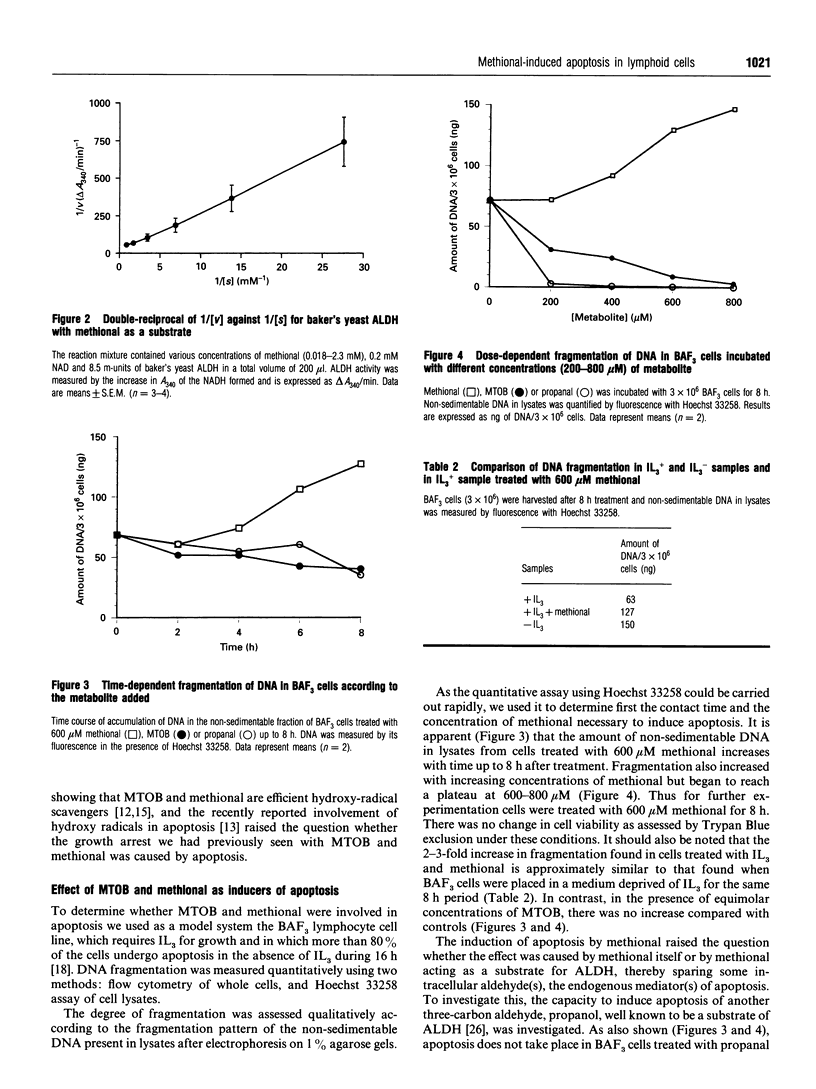
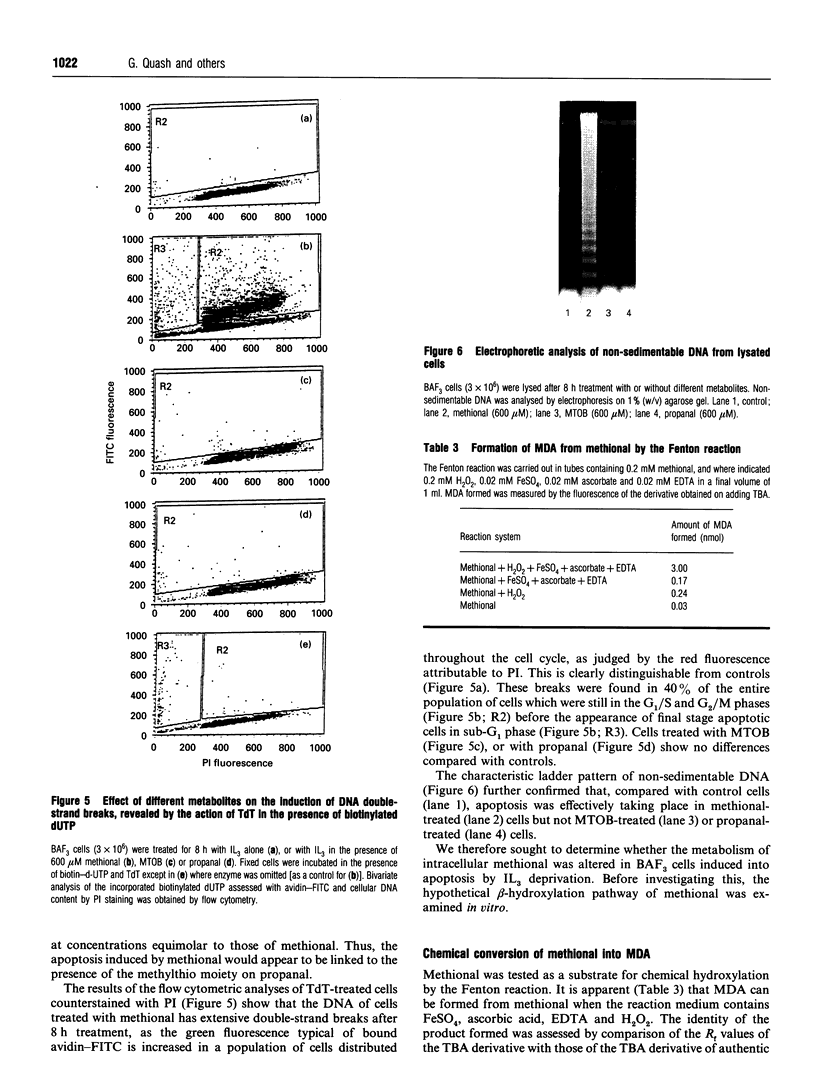


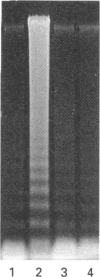
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Chang C. P., Smith R. A. Identification of 2-keto-4-methylthiobutyrate as an intermediate compound in methionine synthesis from 5'-methylthioadenosine. J Biol Chem. 1982 Apr 25;257(8):4196–4202. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Baxter G. D., Lavin M. F. Specific protein dephosphorylation in apoptosis induced by ionizing radiation and heat shock in human lymphoid tumor lines. J Immunol. 1992 Mar 15;148(6):1949–1954. [PubMed] [Google Scholar]
- Blom H. J., Boers G. H., van den Elzen J. P., Gahl W. A., Tangerman A. Transamination of methionine in humans. Clin Sci (Lond) 1989 Jan;76(1):43–49. doi: 10.1042/cs0760043. [DOI] [PubMed] [Google Scholar]
- Bursch W., Oberhammer F., Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci. 1992 Jun;13(6):245–251. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- Bursch W., Paffe S., Putz B., Barthel G., Schulte-Hermann R. Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis. 1990 May;11(5):847–853. doi: 10.1093/carcin/11.5.847. [DOI] [PubMed] [Google Scholar]
- Cohen G., Cederbaum A. I. Chemical evidence for production of hydroxyl radicals during microsomal electron transfer. Science. 1979 Apr 6;204(4388):66–68. doi: 10.1126/science.432627. [DOI] [PubMed] [Google Scholar]
- Cohen G., Cederbaum A. I. Microsomal metabolism of hydroxyl radical scavenging agents: relationship to the microsomal oxidation of alcohols. Arch Biochem Biophys. 1980 Feb;199(2):438–447. doi: 10.1016/0003-9861(80)90300-8. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Marvel J., Malde P., Lopez-Rivas A. Interleukin 3 protects murine bone marrow cells from apoptosis induced by DNA damaging agents. J Exp Med. 1992 Oct 1;176(4):1043–1051. doi: 10.1084/jem.176.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Humphreys J. S., Reed L. J. A potent, heat-stable protein inhibitor of [branched-chain alpha-keto acid dehydrogenase]-phosphatase from bovine kidney mitochondria. Proc Natl Acad Sci U S A. 1986 Jan;83(2):285–289. doi: 10.1073/pnas.83.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Merryfield M. L., Humphreys J. S., Reed L. J. Purification and properties of branched-chain alpha-keto acid dehydrogenase phosphatase from bovine kidney. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4335–4338. doi: 10.1073/pnas.81.14.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Reed L. J. Purification and characterization of a divalent cation-independent, spermine-stimulated protein phosphatase from bovine kidney mitochondria. J Biol Chem. 1987 Apr 15;262(11):5133–5138. [PubMed] [Google Scholar]
- Deitrich R. A., Bludeau P., Stock T., Roper M. Induction of different rat liver supernatant aldehyde dehydrogenases by phenobarbital and tetrachlorodibenzo-p-dioxin. J Biol Chem. 1977 Sep 10;252(17):6169–6176. [PubMed] [Google Scholar]
- Dickinson F. M., Hart G. J. Kinetic studies of the mechanism of sheep liver cytoplasmic aldehyde dehydrogenase. Prog Clin Biol Res. 1982;114:11–22. [PubMed] [Google Scholar]
- Feinstein E., Canaani E., Weiner L. M. Dependence of nucleic acid degradation on in situ free-radical production by adriamycin. Biochemistry. 1993 Dec 7;32(48):13156–13161. doi: 10.1021/bi00211a026. [DOI] [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993 Apr 15;53(8):1945–1951. [PubMed] [Google Scholar]
- Gutteridge J. M. Identification of malondialdehyde as the TBA-reactant formed by bleomycin-iron free radical damage to DNA. FEBS Lett. 1979 Sep 15;105(2):278–282. doi: 10.1016/0014-5793(79)80629-8. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Paxton R., DePaoli-Roach A. A. Inhibition of branched chain alpha-ketoacid dehydrogenase kinase activity by alpha-chloroisocaproate. J Biol Chem. 1982 Dec 10;257(23):13915–13918. [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Gerok W. Glutamine metabolism in isolated perfused rat liver. The transamination pathway. Biol Chem Hoppe Seyler. 1985 Jun;366(6):527–536. doi: 10.1515/bchm3.1985.366.1.527. [DOI] [PubMed] [Google Scholar]
- Jarvis W. D., Kolesnick R. N., Fornari F. A., Traylor R. S., Gewirtz D. A., Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. M., Yeaman S. J. Oxidative decarboxylation of 4-methylthio-2-oxobutyrate by branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1986 Jul 15;237(2):621–623. doi: 10.1042/bj2370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatani N., Nelson-Rees W. A., Carson D. A. Selective killing of human malignant cell lines deficient in methylthioadenosine phosphorylase, a purine metabolic enzyme. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1219–1223. doi: 10.1073/pnas.78.2.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 1982 Jul 19;144(1):57–62. doi: 10.1016/0014-5793(82)80568-1. [DOI] [PubMed] [Google Scholar]
- Lindahl R., Clark R., Evces S. Histochemical localization of aldehyde dehydrogenase during rat hepatocarcinogenesis. Cancer Res. 1983 Dec;43(12 Pt 1):5972–5977. [PubMed] [Google Scholar]
- Lucas M., Solano F., Sanz A. Induction of programmed cell death (apoptosis) in mature lymphocytes. FEBS Lett. 1991 Feb 11;279(1):19–20. doi: 10.1016/0014-5793(91)80239-y. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Enzymatic preparation of alpha-keto acids. J Biol Chem. 1952 May;197(1):309–317. [PubMed] [Google Scholar]
- Mecham J. O., Rowitch D., Wallace C. D., Stern P. H., Hoffman R. M. The metabolic defect of methionine dependence occurs frequently in human tumor cell lines. Biochem Biophys Res Commun. 1983 Dec 16;117(2):429–434. doi: 10.1016/0006-291x(83)91218-4. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ogier G., Chantepie J., Deshayes C., Chantegrel B., Charlot C., Doutheau A., Quash G. Contribution of 4-methylthio-2-oxobutanoate and its transaminase to the growth of methionine-dependent cells in culture. Effect of transaminase inhibitors. Biochem Pharmacol. 1993 Apr 22;45(8):1631–1644. doi: 10.1016/0006-2952(93)90304-f. [DOI] [PubMed] [Google Scholar]
- Reifsnyder D. H., Young C. T., Jones E. E. The use of low protein liquid diets to determine the methionine requirement and the efficacy of methionine hydroxy analogue for the three-week-old pig. J Nutr. 1984 Sep;114(9):1705–1715. doi: 10.1093/jn/114.9.1705. [DOI] [PubMed] [Google Scholar]
- SHAPIRO S. K., MATHER A. N. The enzymatic decomposition of S-adenosyl-L-methionine. J Biol Chem. 1958 Sep;233(3):631–633. [PubMed] [Google Scholar]
- Steele R. D., Benevenga N. J. Identification of 3-methylthiopropionic acid as an intermediate in mammalian methionine metabolism in vitro. J Biol Chem. 1978 Nov 10;253(21):7844–7850. [PubMed] [Google Scholar]
- Summerfield F. W., Tappel A. L. Cross-linking of DNA in liver and testes of rats fed 1,3-propanediol. Chem Biol Interact. 1984 Jun;50(1):87–96. doi: 10.1016/0009-2797(84)90134-0. [DOI] [PubMed] [Google Scholar]
- Summerfield F. W., Tappel A. L. Determination of malondialdehyde-DNA crosslinks by fluorescence and incorporation of tritium. Anal Biochem. 1981 Feb;111(1):77–82. doi: 10.1016/0003-2697(81)90231-1. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Methylthio group cleavage from methylthioadenosine. Description of an enzyme and its relationship to the methylthio requirement of certain cells in culture. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1273–1280. doi: 10.1016/0006-291x(77)91430-9. [DOI] [PubMed] [Google Scholar]
- Trackman P. C., Abeles R. H. Methionine synthesis from 5'-S-Methylthioadenosine. Resolution of enzyme activities and identification of 1-phospho-5-S methylthioribulose. J Biol Chem. 1983 Jun 10;258(11):6717–6720. [PubMed] [Google Scholar]
- Wu G. Y., Thompson J. R. Is methionine transaminated in skeletal muscle? Biochem J. 1989 Jan 1;257(1):281–284. doi: 10.1042/bj2570281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J. The 2-oxo acid dehydrogenase complexes: recent advances. Biochem J. 1989 Feb 1;257(3):625–632. doi: 10.1042/bj2570625. [DOI] [PMC free article] [PubMed] [Google Scholar]