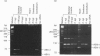Abstract
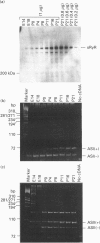
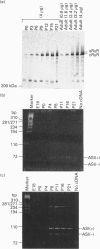
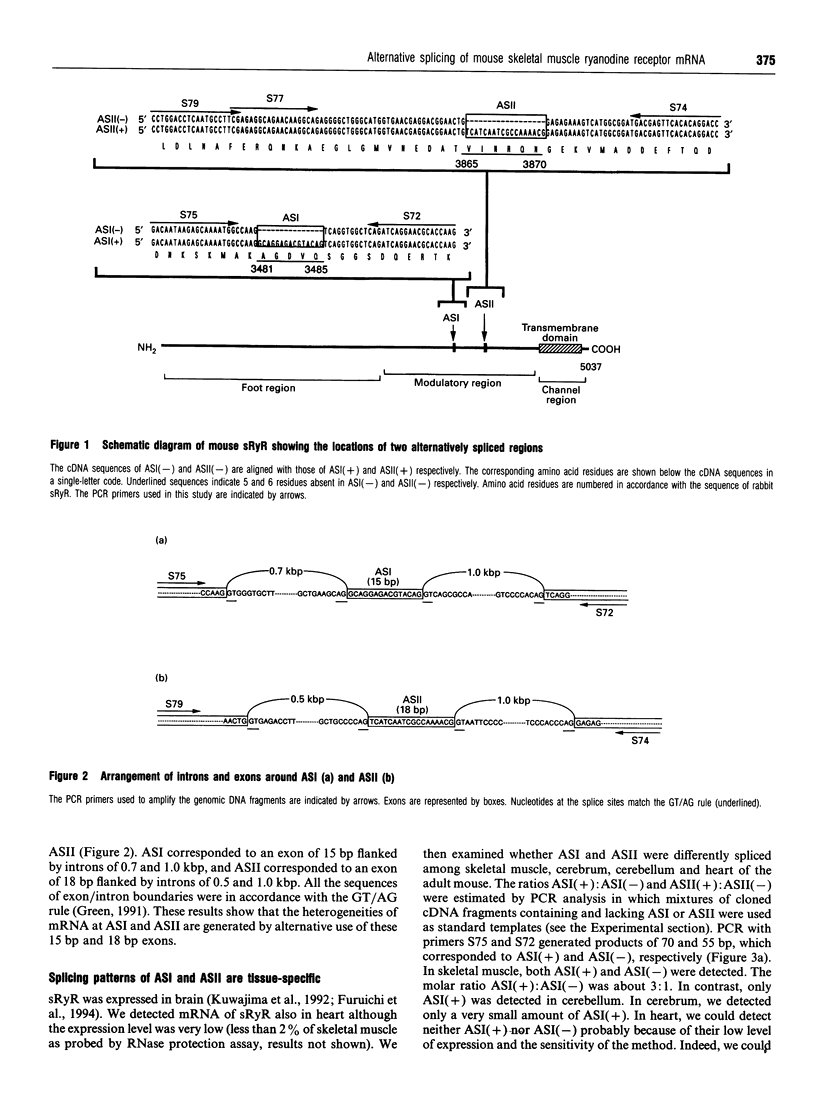
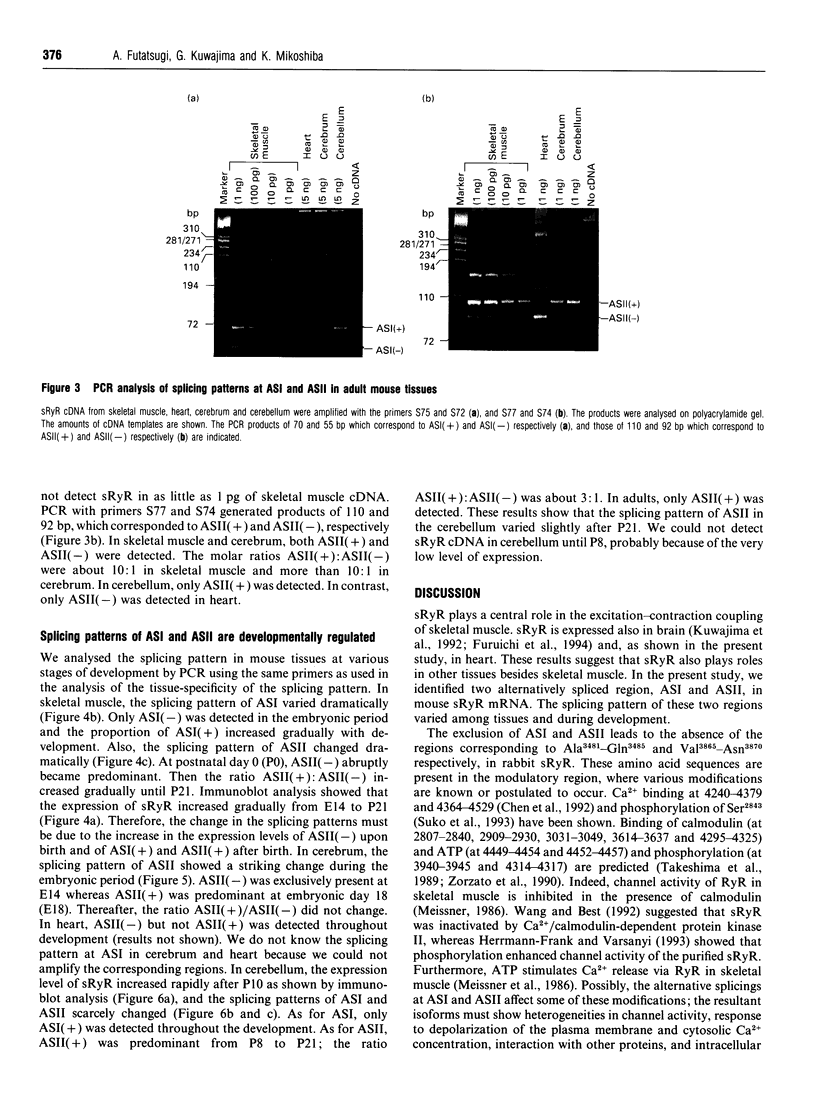
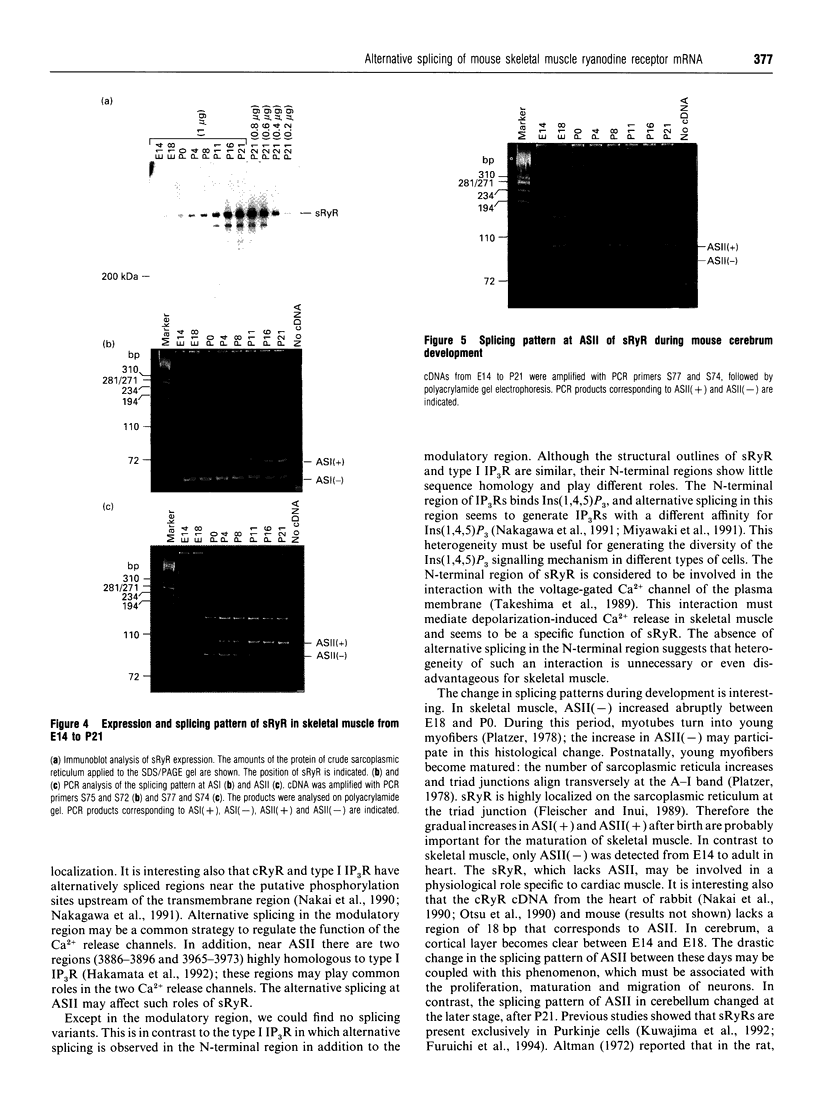
The ryanodine receptor is a channel for Ca2+ release from intracellular stores. By PCR analysis, we identified two alternatively spliced regions in mRNA of the mouse skeletal muscle ryanodine receptor (sRyR). The splice variants were characterized by the presence or absence of 15 bp (ASI) and 18 bp (ASII) exons. The exclusion of these exons results in the absence of the regions corresponding to Ala3481-Gln3485 and Val3865-Asn3870, respectively, of rabbit sRyR; these amino acid sequences exist in the modulatory region, where sites for phosphorylation and binding of Ca2+, calmodulin and ATP are postulated to be. We also detected sRyR in brain and heart as well as in skeletal muscle, and the splicing patterns were found to be tissue-specific. Only the ASII-lacking isoform was detected in heart, whereas in other tissues the ASII-containing isoform was predominant. The splicing patterns were also found to change during development. In skeletal muscle, the ASI-containing isoform increased gradually from embryo to adult. The ASII-lacking isoform abruptly increased upon birth, but the ASII-containing isoform increased steadily afterwards. In cerebrum, the ratio of the ASII-containing isoform to the ASII-lacking one increased abruptly during embryonic days 14 and 18. These findings suggest that the alternative splicing of ASI and ASII, by affecting the modulatory region, generates functionally different sRyR isoforms in a tissue-specific and developmentally regulated manner.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Chen S. R., Zhang L., MacLennan D. H. Characterization of a Ca2+ binding and regulatory site in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1992 Nov 15;267(32):23318–23326. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Furutama D., Hakamata Y., Nakai J., Takeshima H., Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci. 1994 Aug;14(8):4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini G., Clementi E., Ceci R., Marziali G., Sorrentino V. Expression of a ryanodine receptor-Ca2+ channel that is regulated by TGF-beta. Science. 1992 Jul 3;257(5066):91–94. doi: 10.1126/science.1320290. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Hakamata Y., Nakai J., Takeshima H., Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992 Nov 9;312(2-3):229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A., Varsányi M. Enhancement of Ca2+ release channel activity by phosphorylation of the skeletal muscle ryanodine receptor. FEBS Lett. 1993 Oct 18;332(3):237–242. doi: 10.1016/0014-5793(93)80640-g. [DOI] [PubMed] [Google Scholar]
- Kuwajima G., Futatsugi A., Niinobe M., Nakanishi S., Mikoshiba K. Two types of ryanodine receptors in mouse brain: skeletal muscle type exclusively in Purkinje cells and cardiac muscle type in various neurons. Neuron. 1992 Dec;9(6):1133–1142. doi: 10.1016/0896-6273(92)90071-k. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. Characterization of the major brain form of the ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Sep 15;268(26):19785–19790. [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Meissner G. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry. 1986 Jan 14;25(1):244–251. doi: 10.1021/bi00349a034. [DOI] [PubMed] [Google Scholar]
- Miyawaki A., Furuichi T., Ryou Y., Yoshikawa S., Nakagawa T., Saitoh T., Mikoshiba K. Structure-function relationships of the mouse inositol 1,4,5-trisphosphate receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4911–4915. doi: 10.1073/pnas.88.11.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros L. G., Bak J., Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature. 1993 Jul 1;364(6432):76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Okano H., Furuichi T., Aruga J., Mikoshiba K. The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally specific manner. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J., Imagawa T., Hakamat Y., Shigekawa M., Takeshima H., Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990 Oct 1;271(1-2):169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Kuwajima G., Mikoshiba K. Immunohistochemical localization of ryanodine receptors in mouse central nervous system. Neurosci Res. 1992 Oct;15(1-2):130–142. doi: 10.1016/0168-0102(92)90026-9. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Platzer A. C. The ultrastructure of normal myogenesis in the limb of the mouse. Anat Rec. 1978 Mar;190(3):639–657. doi: 10.1002/ar.1091900303. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Schell M. J., Danoff S. K., Ross C. A. Inositol (1,4,5)-trisphosphate receptor: characterization of neuron-specific alternative splicing in rat brain and peripheral tissues. Brain Res Mol Brain Res. 1993 Mar;17(3-4):212–216. doi: 10.1016/0169-328x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Suko J., Maurer-Fogy I., Plank B., Bertel O., Wyskovsky W., Hohenegger M., Hellmann G. Phosphorylation of serine 2843 in ryanodine receptor-calcium release channel of skeletal muscle by cAMP-, cGMP- and CaM-dependent protein kinase. Biochim Biophys Acta. 1993 Jan 17;1175(2):193–206. doi: 10.1016/0167-4889(93)90023-i. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- White A. M., Watson S. P., Galione A. Cyclic ADP-ribose-induced Ca2+ release from rat brain microsomes. FEBS Lett. 1993 Mar 8;318(3):259–263. doi: 10.1016/0014-5793(93)80524-x. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]