Abstract
Overexpression of pp60c-src in mouse fibroblasts potentiates both agonist-induced signalling through beta-adrenergic receptors and cyclic AMP accumulation in response to cholera toxin [Bushman, Wilson, Luttrell, Moyers and Parsons (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 7462-7466; Moyers, Bouton and Parsons (1993) Mol. Cell. Biol. 13, 2391-2400]. In reconstitution experiments in vitro, phosphorylation of Gs alpha by immune-complexed pp60c-src resulted in enhanced rates of receptor-mediated guanosine 5'-[gamma-thio]triphosphate (GTP[S]) binding and GTP hydrolysis [Hausdorff, Pitcher, Luttrell, Linder, Kurose, Parsons, Caron and Lefkowitz (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 5720-5724]. These results suggest that one mechanism by which pp60c-src affects signalling through the beta-adrenergic receptor is by phosphorylation and functional alteration of the G protein. To elucidate how phosphorylation of Gs alpha might affect its function, we subjected phosphorylated, recombinant Gs alpha to tryptic phosphopeptide analysis. Phosphotryptic peptides were purified by h.p.l.c. and analysed by Edman degradation to determine the cycle numbers at which radiolabelled phosphotyrosine was released. Candidate peptides that contained Tyr residues at the corresponding positions were synthesized, phosphorylated in vitro by pp60c-src, and their migrations in two-dimensional electrophoresis/t.l.c. were compared with those of tryptic phosphopeptides from intact Gs alpha. We report here that Gs alpha is phosphorylated on two residues by pp60c-src, namely, Tyr-37 and Tyr-377. Tyr-37 lies near the site of beta gamma binding in the N-terminus, within a region postulated to modulate GDP dissociation and activation by GTP [Johnson, Dhanasekaran, Gupta, Lowndes, Vaillancourt and Ruoho (1991) J. Cell Biochem. 47, 136-146], while Tyr-377 is located in the extreme C-terminus, within a region of Gs alpha important for receptor interaction [Sullivan, Miller, Masters, Beiderman, Heideman and Bourne (1987) Nature (London) 334, 712-715]. The location of these residues suggests that phosphorylation may affect the function of both of these regulatory domains.
Full text
PDF


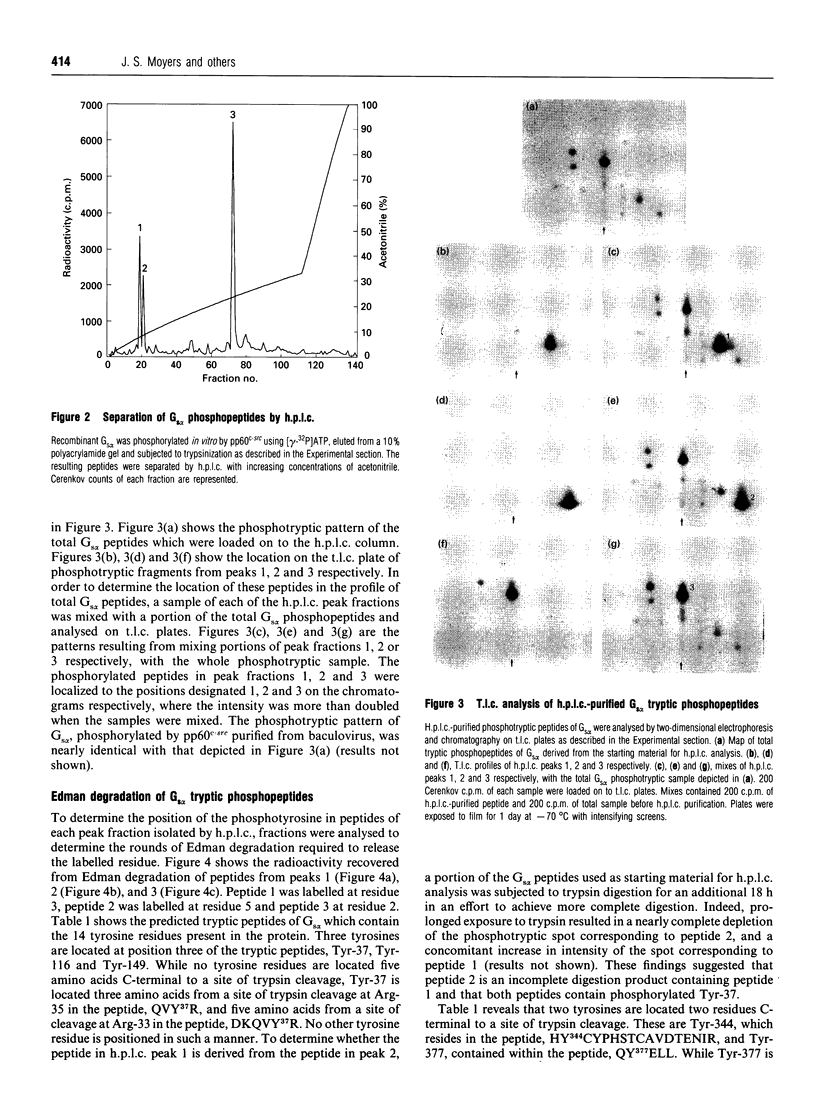
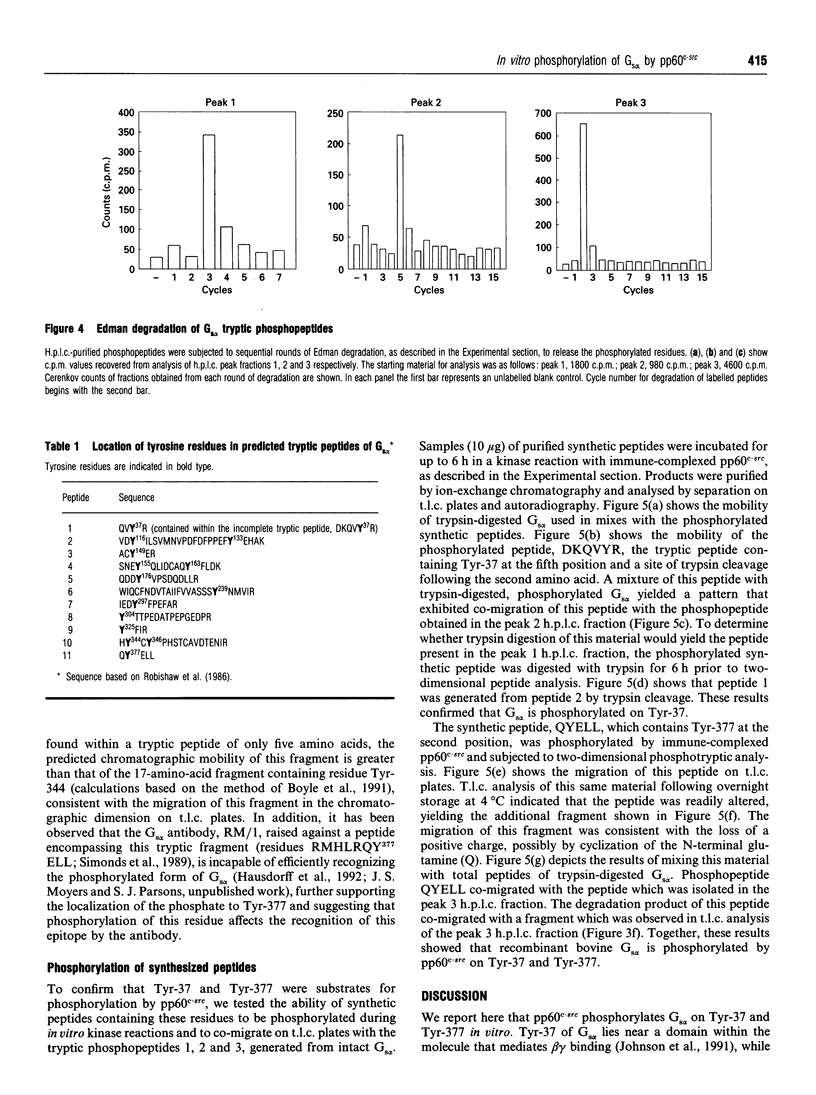
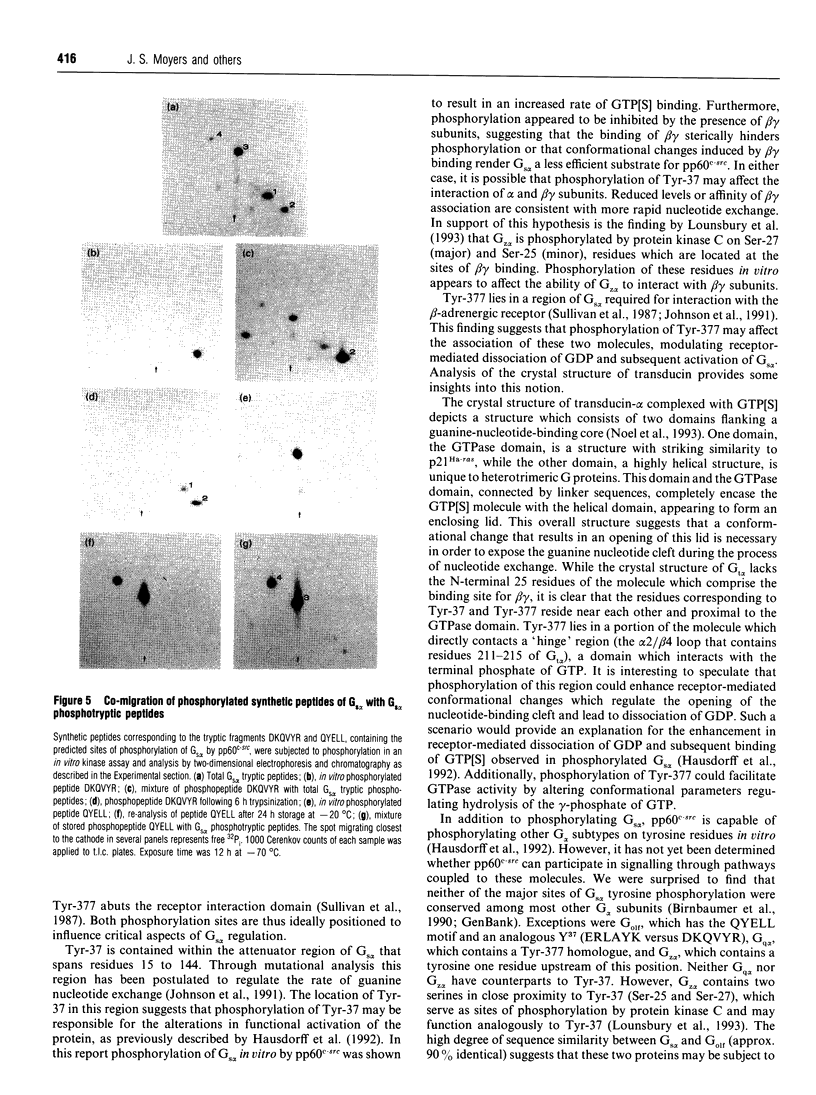

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiho H., Tokumitsu Y., Noda M., Nomura Y. Decrease in coupling of Gs in v-src-transformed NIH-3T3 fibroblasts: possible involvement of tyrosine phosphorylation of Gs by pp60v-src. Arch Biochem Biophys. 1993 Jul;304(1):235–241. doi: 10.1006/abbi.1993.1344. [DOI] [PubMed] [Google Scholar]
- Alexandropoulos K., Joseph C. K., Spangler R., Foster D. A. Evidence that a G-protein transduces signals initiated by the protein-tyrosine kinase v-Fps. J Biol Chem. 1991 Aug 25;266(24):15583–15586. [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Bushman W. A., Wilson L. K., Luttrell D. K., Moyers J. S., Parsons S. J. Overexpression of c-src enhances beta-adrenergic-induced cAMP accumulation. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7462–7466. doi: 10.1073/pnas.87.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J. M., Pappin D. J., Mark J., Aebersold R., Köster H. Functionalized membrane supports for covalent protein microsequence analysis. Anal Biochem. 1991 Apr;194(1):110–120. doi: 10.1016/0003-2697(91)90157-o. [DOI] [PubMed] [Google Scholar]
- Crouch M. F. Growth factor-induced cell division is paralleled by translocation of Gi alpha to the nucleus. FASEB J. 1991 Feb;5(2):200–206. doi: 10.1096/fasebj.5.2.1900794. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Schomisch S. J., Kusner D. J., Xie M. Phospholipase D activity in phagocytic leucocytes is synergistically regulated by G-protein- and tyrosine kinase-based mechanisms. Biochem J. 1993 May 15;292(Pt 1):121–128. doi: 10.1042/bj2920121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M. P., Freissmuth M., Gilman A. G. Expression of Gs alpha in Escherichia coli. Purification and properties of two forms of the protein. J Biol Chem. 1989 Jan 5;264(1):409–418. [PubMed] [Google Scholar]
- Hausdorff W. P., Pitcher J. A., Luttrell D. K., Linder M. E., Kurose H., Parsons S. J., Caron M. G., Lefkowitz R. J. Tyrosine phosphorylation of G protein alpha subunits by pp60c-src. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L., Dhanasekaran N., Gupta S. K., Lowndes J. M., Vaillancourt R. R., Ruoho A. E. Genetic and structural analysis of G protein alpha subunit regulatory domains. J Cell Biochem. 1991 Oct;47(2):136–146. doi: 10.1002/jcb.240470207. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J., Rajaram R., Lakonishok M., Benovic J. L., Cerione R. A. Insulin-dependent phosphorylation of GTP-binding proteins in phospholipid vesicles. J Biol Chem. 1988 Sep 5;263(25):12333–12341. [PubMed] [Google Scholar]
- Lee E., Linder M. E., Gilman A. G. Expression of G-protein alpha subunits in Escherichia coli. Methods Enzymol. 1994;237:146–164. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- Liang M. N., Garrison J. C. The epidermal growth factor receptor is coupled to a pertussis toxin-sensitive guanine nucleotide regulatory protein in rat hepatocytes. J Biol Chem. 1991 Jul 15;266(20):13342–13349. [PubMed] [Google Scholar]
- Linder M. E., Middleton P., Hepler J. R., Taussig R., Gilman A. G., Mumby S. M. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury K. M., Schlegel B., Poncz M., Brass L. F., Manning D. R. Analysis of Gz alpha by site-directed mutagenesis. Sites and specificity of protein kinase C-dependent phosphorylation. J Biol Chem. 1993 Feb 15;268(5):3494–3498. [PubMed] [Google Scholar]
- Luttrell L., Kilgour E., Larner J., Romero G. A pertussis toxin-sensitive G-protein mediates some aspects of insulin action in BC3H-1 murine myocytes. J Biol Chem. 1990 Oct 5;265(28):16873–16879. [PubMed] [Google Scholar]
- Mattingly R. R., Wasilenko W. J., Woodring P. J., Garrison J. C. Selective amplification of endothelin-stimulated inositol 1,4,5-trisphosphate and calcium signaling by v-src transformation of rat-1 fibroblasts. J Biol Chem. 1992 Apr 15;267(11):7470–7477. [PubMed] [Google Scholar]
- Meyer H. E., Hoffmann-Posorske E., Donella-Deana A., Korte H. Sequence analysis of phosphotyrosine-containing peptides. Methods Enzymol. 1991;201:206–224. doi: 10.1016/0076-6879(91)01019-x. [DOI] [PubMed] [Google Scholar]
- Miller R. T., Masters S. B., Sullivan K. A., Beiderman B., Bourne H. R. A mutation that prevents GTP-dependent activation of the alpha chain of Gs. Nature. 1988 Aug 25;334(6184):712–715. doi: 10.1038/334712a0. [DOI] [PubMed] [Google Scholar]
- Moyers J. S., Bouton A. H., Parsons S. J. The sites of phosphorylation by protein kinase C and an intact SH2 domain are required for the enhanced response to beta-adrenergic agonists in cells overexpressing c-src. Mol Cell Biol. 1993 Apr;13(4):2391–2400. doi: 10.1128/mcb.13.4.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. G., Rashed H. M., Patel T. B. Epidermal growth factor stimulates rat cardiac adenylate cyclase through a GTP-binding regulatory protein. Biochem J. 1989 Dec 1;264(2):563–571. doi: 10.1042/bj2640563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J. P., Hamm H. E., Sigler P. B. The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature. 1993 Dec 16;366(6456):654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Smigel M. D., Gilman A. G. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem. 1986 Jul 25;261(21):9587–9590. [PubMed] [Google Scholar]
- Rothenberg P. L., Kahn C. R. Insulin inhibits pertussis toxin-catalyzed ADP-ribosylation of G-proteins. Evidence for a novel interaction between insulin receptors and G-proteins. J Biol Chem. 1988 Oct 25;263(30):15546–15552. [PubMed] [Google Scholar]
- Russo G. L., Vandenberg M. T., Yu I. J., Bae Y. S., Franza B. R., Jr, Marshak D. R. Casein kinase II phosphorylates p34cdc2 kinase in G1 phase of the HeLa cell division cycle. J Biol Chem. 1992 Oct 5;267(28):20317–20325. [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson M. S., Herman W. H. Protein kinase C and protein tyrosine kinase activity contribute to mitogenic signaling by endothelin-1. Cross-talk between G protein-coupled receptors and pp60c-src. J Biol Chem. 1993 May 5;268(13):9347–9357. [PubMed] [Google Scholar]
- Stokoe D., Campbell D. G., Nakielny S., Hidaka H., Leevers S. J., Marshall C., Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992 Nov;11(11):3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum I. The epidermal growth factor receptor is coupled to a phospholipase A2-specific pertussis toxin-inhibitable guanine nucleotide-binding regulatory protein in cultured rat inner medullary collecting tubule cells. J Biol Chem. 1990 Mar 15;265(8):4218–4222. [PubMed] [Google Scholar]
- Torti M., Crouch M. F., Lapetina E. G. Epinephrine induces association of pp60src with Gi alpha in human platelets. Biochem Biophys Res Commun. 1992 Jul 15;186(1):440–447. doi: 10.1016/s0006-291x(05)80827-7. [DOI] [PubMed] [Google Scholar]
- Wasilenko W. J., Payne D. M., Fitzgerald D. L., Weber M. J. Phosphorylation and activation of epidermal growth factor receptors in cells transformed by the src oncogene. Mol Cell Biol. 1991 Jan;11(1):309–321. doi: 10.1128/mcb.11.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. K., Luttrell D. K., Parsons J. T., Parsons S. J. pp60c-src tyrosine kinase, myristylation, and modulatory domains are required for enhanced mitogenic responsiveness to epidermal growth factor seen in cells overexpressing c-src. Mol Cell Biol. 1989 Apr;9(4):1536–1544. doi: 10.1128/mcb.9.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick Y., Sagi-Eisenberg R., Pines M., Gierschik P., Spiegel A. M. Multisite phosphorylation of the alpha subunit of transducin by the insulin receptor kinase and protein kinase C. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9294–9297. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]