Abstract
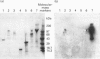
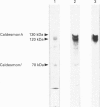
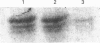
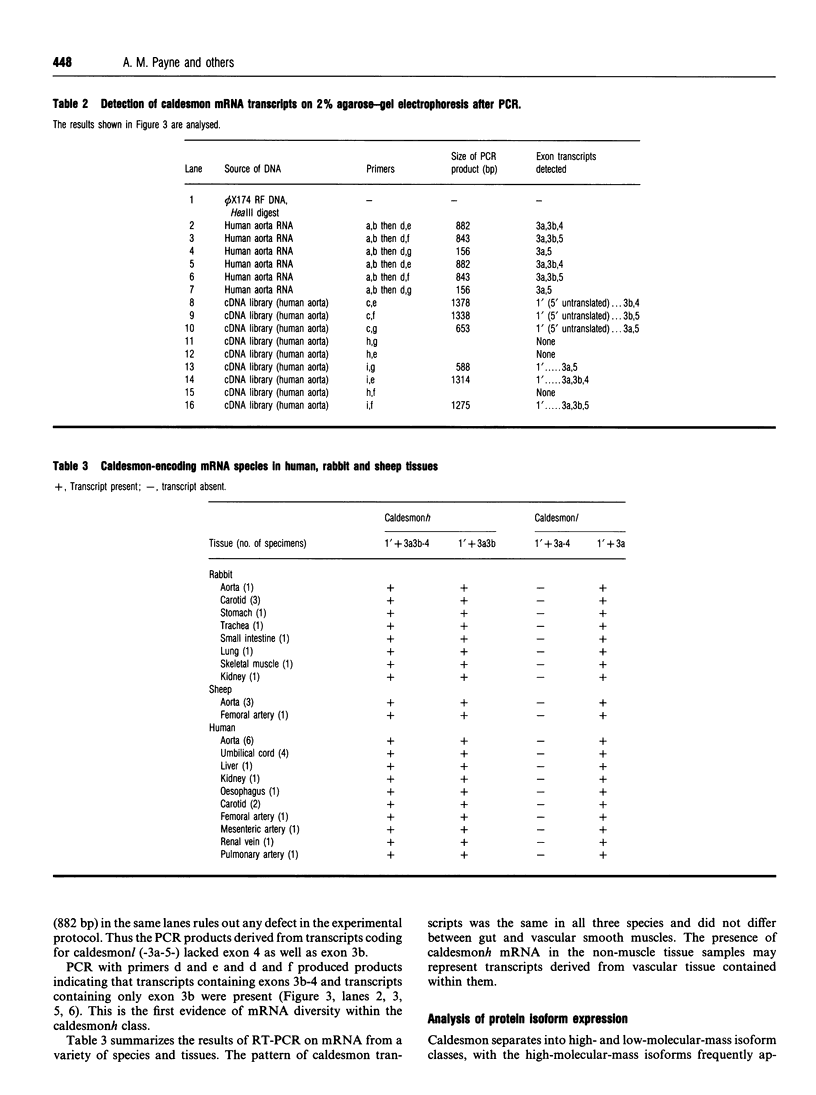
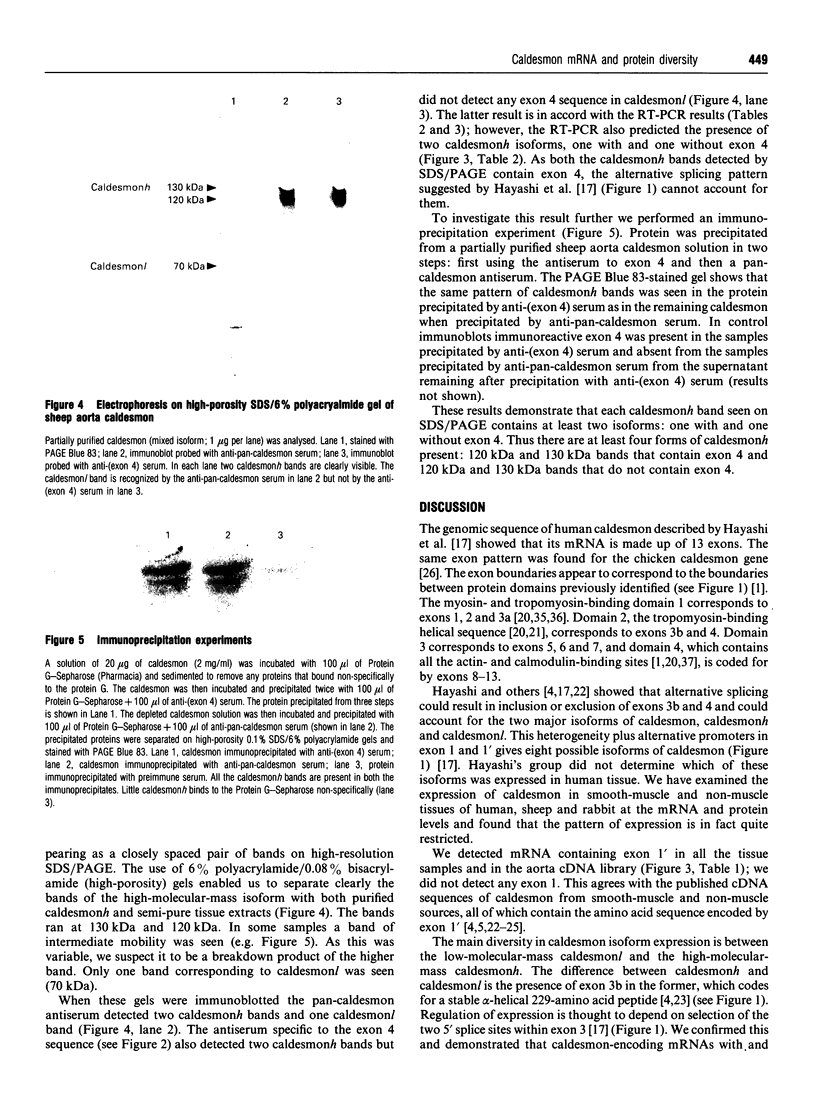
The recent determination of the genomic sequence of human caldesmon indicates that eight caldesmon mRNA species could be generated by selection of exon 1 or 1', exon 3a or 3ab and/or exon 4. We used reverse transcriptase PCR to determine which transcripts were produced in human, rabbit and sheep artery, vein, lung, intestine, kidney and liver. In all tissues the same three transcripts were present: exons 1'-2-3a-5-6...13, exons 1'-2-3a3b-5-6-...13 and exons 1'-2-3a3b-4-5-6...13. Exon 1 was not present and exon 4 was only present when exon 3b was also present. Three protein isoforms of caldesmon can be distinguished by electrophoresis on high-porosity 6% polyacrylamide gel: 130 kDa, 120 kDa and 70 kDa. The 70 kDa isoform lacks the sequence encoded by exon 3b. We investigated whether the two high-molecular-mass isoforms correspond to the presence and absence of exon 4 using an antiserum specific to the sequence encoded by exon 4. Western-blotting and immunoprecipitation experiments showed that both the 130 kDa and the 120 kDa isoforms were expressed with and without the exon 4 sequence. We therefore propose that the molecular-mass heterogeneity arises from additional first exons, possibly with separate promoter regions, which have not yet been characterized in the genomic sequence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogatcheva N. V., Vorotnikov A. V., Birukov K. G., Shirinsky V. P., Gusev N. B. Phosphorylation by casein kinase II affects the interaction of caldesmon with smooth muscle myosin and tropomyosin. Biochem J. 1993 Mar 1;290(Pt 2):437–442. doi: 10.1042/bj2900437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Bryan J., Lee R. Sequence of an avian non-muscle caldesmon. J Muscle Res Cell Motil. 1991 Aug;12(4):372–375. doi: 10.1007/BF01738592. [DOI] [PubMed] [Google Scholar]
- Cuda G., Fananapazir L., Zhu W. S., Sellers J. R., Epstein N. D. Skeletal muscle expression and abnormal function of beta-myosin in hypertrophic cardiomyopathy. J Clin Invest. 1993 Jun;91(6):2861–2865. doi: 10.1172/JCI116530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid M. G., Shekhonin B. V., Koteliansky V. E., Glukhova M. A. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol. 1992 Oct;153(2):185–193. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Koteliansky V. E. Developmental changes in expression of contractile and cytoskeletal proteins in human aortic smooth muscle. J Biol Chem. 1990 Aug 5;265(22):13042–13046. [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Frid M. G., Ornatsky O. I., Belkin A. M., Mukhin D. N., Orekhov A. N., Koteliansky V. E., Smirnov V. N. Modulation of human aorta smooth muscle cell phenotype: a study of muscle-specific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9542–9546. doi: 10.1073/pnas.85.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Ornatsky O. I., Vasilevskaya T. D., Koteliansky V. E., Smirnov V. N. Immunoreactive forms of caldesmon in cultivated human vascular smooth muscle cells. FEBS Lett. 1987 Jun 29;218(2):292–294. doi: 10.1016/0014-5793(87)81064-5. [DOI] [PubMed] [Google Scholar]
- Haruna M., Hayashi K., Yano H., Takeuchi O., Sobue K. Common structural and expressional properties of vertebrate caldesmon genes. Biochem Biophys Res Commun. 1993 Nov 30;197(1):145–153. doi: 10.1006/bbrc.1993.2453. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Fujio Y., Kato I., Sobue K. Structural and functional relationships between h- and l-caldesmons. J Biol Chem. 1991 Jan 5;266(1):355–361. [PubMed] [Google Scholar]
- Hayashi K., Kanda K., Kimizuka F., Kato I., Sobue K. Primary structure and functional expression of h-caldesmon complementary DNA. Biochem Biophys Res Commun. 1989 Oct 16;164(1):503–511. doi: 10.1016/0006-291x(89)91748-8. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Yano H., Hashida T., Takeuchi R., Takeda O., Asada K., Takahashi E., Kato I., Sobue K. Genomic structure of the human caldesmon gene. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12122–12126. doi: 10.1073/pnas.89.24.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Characterization of caldesmon binding to myosin. J Biol Chem. 1990 Nov 15;265(32):19672–19678. [PMC free article] [PubMed] [Google Scholar]
- Humphrey M. B., Herrera-Sosa H., Gonzalez G., Lee R., Bryan J. Cloning of cDNAs encoding human caldesmons. Gene. 1992 Mar 15;112(2):197–204. doi: 10.1016/0378-1119(92)90376-z. [DOI] [PubMed] [Google Scholar]
- Lehman W., Denault D., Marston S. The caldesmon content of vertebrate smooth muscle. Biochim Biophys Acta. 1993 Nov 10;1203(1):53–59. doi: 10.1016/0167-4838(93)90035-p. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Wang C. L. Electron microscopic studies of chicken gizzard caldesmon and its complex with calmodulin. J Muscle Res Cell Motil. 1991 Apr;12(2):145–151. doi: 10.1007/BF01774033. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. The molecular anatomy of caldesmon. Biochem J. 1991 Oct 1;279(Pt 1):1–16. doi: 10.1042/bj2790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S., Pinter K., Bennett P. Caldesmon binds to smooth muscle myosin and myosin rod and crosslinks thick filaments to actin filaments. J Muscle Res Cell Motil. 1992 Apr;13(2):206–218. doi: 10.1007/BF01874158. [DOI] [PubMed] [Google Scholar]
- Novy R. E., Lin J. L., Lin J. J. Characterization of cDNA clones encoding a human fibroblast caldesmon isoform and analysis of caldesmon expression in normal and transformed cells. J Biol Chem. 1991 Sep 5;266(25):16917–16924. [PubMed] [Google Scholar]
- Redwood C. S., Marston S. B. Binding and regulatory properties of expressed functional domains of chicken gizzard smooth muscle caldesmon. J Biol Chem. 1993 May 25;268(15):10969–10976. [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Sellers J. R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991 Jul 5;266(19):12115–12118. [PubMed] [Google Scholar]
- Stafford W. F., Jancso A., Graceffa P. Caldesmon from rabbit liver: molecular weight and length by analytical ultracentrifugation. Arch Biochem Biophys. 1990 Aug 15;281(1):66–69. doi: 10.1016/0003-9861(90)90413-s. [DOI] [PubMed] [Google Scholar]
- Szpacenko A., Dabrowska R. Functional domain of caldesmon. FEBS Lett. 1986 Jul 7;202(2):182–186. doi: 10.1016/0014-5793(86)80683-4. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Watanabe T., Nakamura N., Sobue K. Morphological and biochemical analyses of contractile proteins (actin, myosin, caldesmon and tropomyosin) in normal and transformed cells. J Cell Sci. 1993 Feb;104(Pt 2):595–606. doi: 10.1242/jcs.104.2.595. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki N., Sobue K., Kanda K., Hada T., Higashino K. Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9049–9053. doi: 10.1073/pnas.84.24.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Chalovich J. M., Graceffa P., Lu R. C., Mabuchi K., Stafford W. F. A long helix from the central region of smooth muscle caldesmon. J Biol Chem. 1991 Jul 25;266(21):13958–13963. [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Matsumura F. Mitosis-specific phosphorylation of caldesmon: possible molecular mechanism of cell rounding during mitosis. Bioessays. 1991 Nov;13(11):563–568. doi: 10.1002/bies.950131103. [DOI] [PubMed] [Google Scholar]