Abstract
Ultraviolet radiation (UVR) induces melanin synthesis by human epidermal melanocytes, and phospholipid-derived 1,2-diacylglycerols (DAGs) have been implicated in mediating this response. In previous experiments, addition of the synthetic DAG 1-oleoyl-2-acetylglycerol to cultured pigment cells stimulated melanogenesis. The purpose of the present study was to analyse the effects of UVR on the endogenous generation of DAGs. It was found that in a number of cultured cell types, including human melanocytes and B16 mouse melanoma cells, but also human keratinocytes and Swiss 3T3 fibroblasts, exposure to a single dose of UVR stimulated a biphasic increase in endogenous DAG formation. An early transient rise, over seconds, was followed by a more sustained delayed rise over minutes. The early rise in DAG levels was accompanied by a transient rise in inositol trisphosphate formation, indicating activation of phosphatidylinositol-specific phospholipase C. The delayed rise was accompanied by activation of phospholipase D. This endogenous DAG formation by pigment cells is further evidence for the involvement of DAGs in UVR-induced epidermal melanin synthesis. Since DAG formation is also seen in other cells types, it is possible that DAGs may be involved in an array of UVR-induced responses.
Full text
PDF

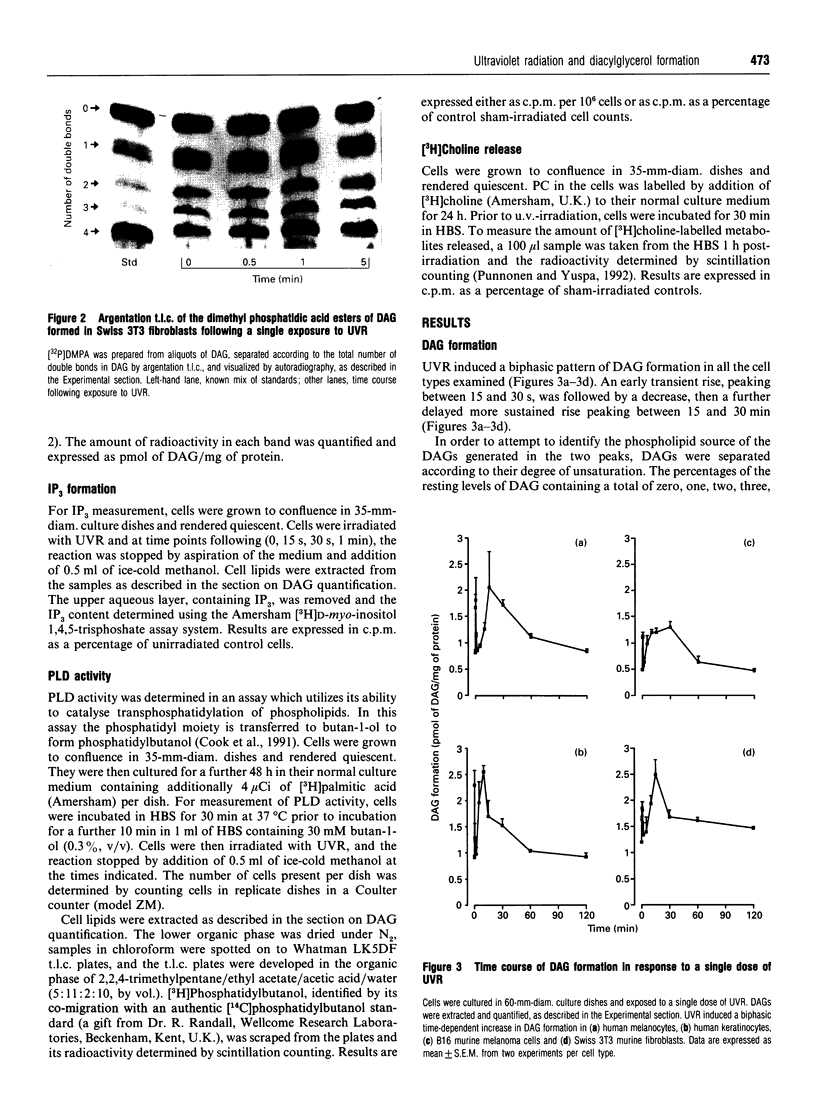
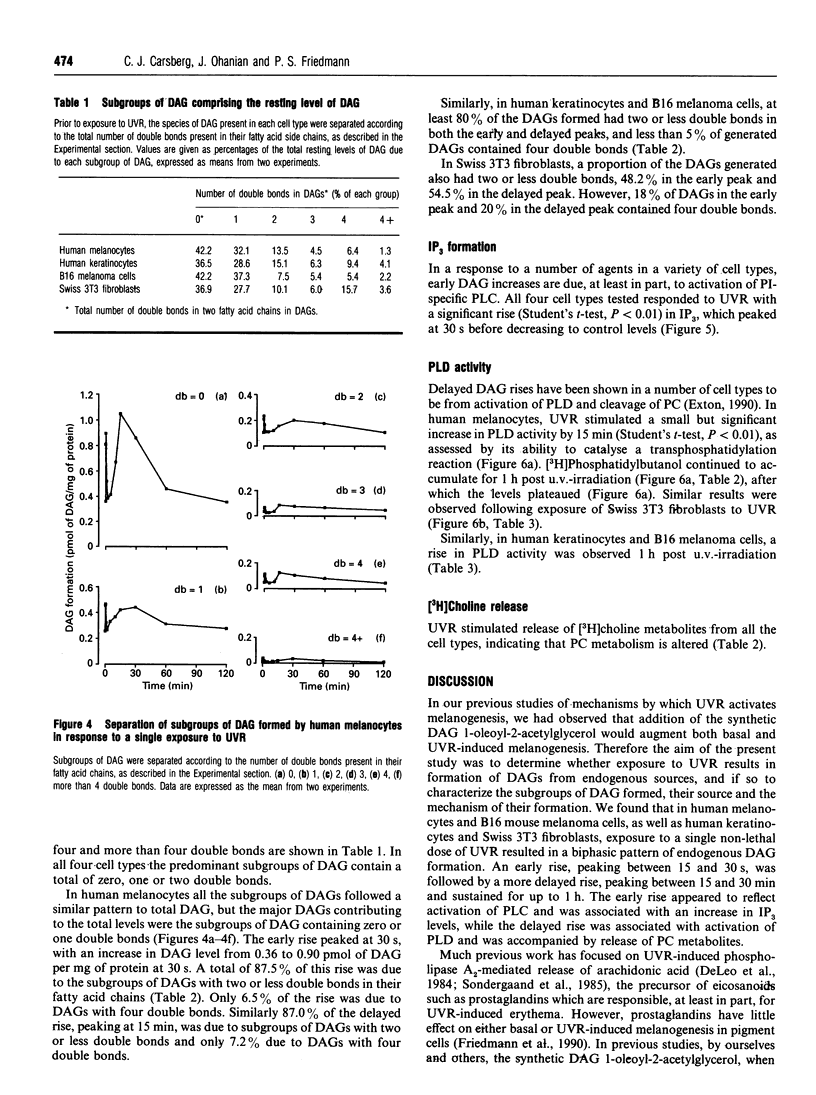
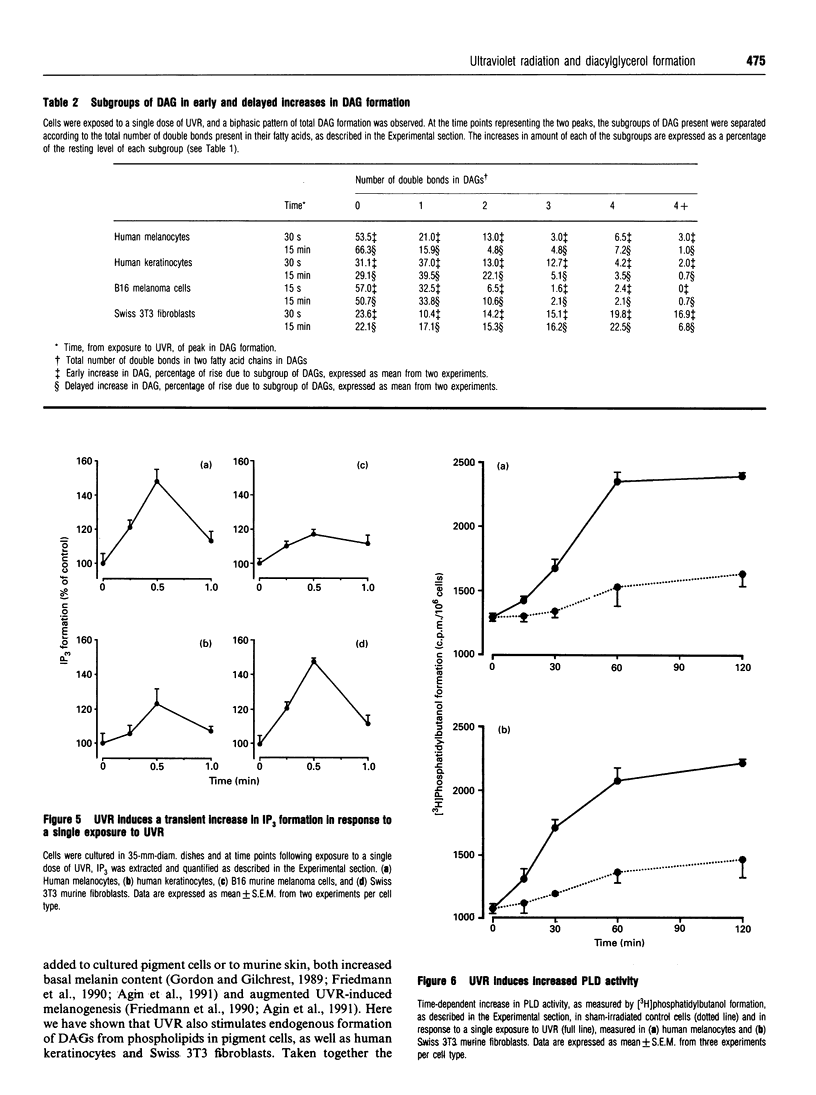


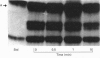
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agin P. P., Dowdy J. C., Costlow M. E. Diacylglycerol-induced melanogenesis in Skh-2 pigmented hairless mice. Photodermatol Photoimmunol Photomed. 1991 Apr;8(2):51–56. [PubMed] [Google Scholar]
- Agwu D. E., McPhail L. C., Chabot M. C., Daniel L. W., Wykle R. L., McCall C. E. Choline-linked phosphoglycerides. A source of phosphatidic acid and diglycerides in stimulated neutrophils. J Biol Chem. 1989 Jan 25;264(3):1405–1413. [PubMed] [Google Scholar]
- Asaoka Y., Nakamura S., Yoshida K., Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992 Oct;17(10):414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsberg C. J., Warenius H. M., Friedmann P. S. Ultraviolet radiation-induced melanogenesis in human melanocytes. Effects of modulating protein kinase C. J Cell Sci. 1994 Sep;107(Pt 9):2591–2597. doi: 10.1242/jcs.107.9.2591. [DOI] [PubMed] [Google Scholar]
- Choudhury G. G., Sylvia V. L., Sakaguchi A. Y. Activation of a phosphatidylcholine-specific phospholipase C by colony stimulating factor 1 receptor requires tyrosine phosphorylation and a guanine nucleotide-binding protein. J Biol Chem. 1991 Dec 5;266(34):23147–23151. [PubMed] [Google Scholar]
- Cook S. J., Briscoe C. P., Wakelam M. J. The regulation of phospholipase D activity and its role in sn-1,2-diradylglycerol formation in bombesin- and phorbol 12-myristate 13-acetate-stimulated Swiss 3T3 cells. Biochem J. 1991 Dec 1;280(Pt 2):431–438. doi: 10.1042/bj2800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. The formation of phosphatidylglycerol and other phospholipids by the transferase activity of phospholipase D. Biochem J. 1967 Jan;102(1):205–210. doi: 10.1042/bj1020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo V. A., Horlick H., Hanson D., Eisinger M., Harber L. C. Ultraviolet radiation induces changes in membrane metabolism of human keratinocytes in culture. J Invest Dermatol. 1984 Nov;83(5):323–326. doi: 10.1111/1523-1747.ep12264114. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Friedmann P. S., Wren F. E., Matthews J. N. Ultraviolet stimulated melanogenesis by human melanocytes is augmented by di-acyl glycerol but not TPA. J Cell Physiol. 1990 Feb;142(2):334–341. doi: 10.1002/jcp.1041420216. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A., Vrabel M. A., Flynn E., Szabo G. Selective cultivation of human melanocytes from newborn and adult epidermis. J Invest Dermatol. 1984 Nov;83(5):370–376. doi: 10.1111/1523-1747.ep12264638. [DOI] [PubMed] [Google Scholar]
- Gordon P. R., Gilchrest B. A. Human melanogenesis is stimulated by diacylglycerol. J Invest Dermatol. 1989 Nov;93(5):700–702. doi: 10.1111/1523-1747.ep12319900. [DOI] [PubMed] [Google Scholar]
- Kennerly D. A. Diacylglycerol metabolism in mast cells. Analysis of lipid metabolic pathways using molecular species analysis of intermediates. J Biol Chem. 1987 Dec 5;262(34):16305–16313. [PubMed] [Google Scholar]
- Kobayashi M., Kanfer J. N. Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J Neurochem. 1987 May;48(5):1597–1603. doi: 10.1111/j.1471-4159.1987.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Liscovitch M. Crosstalk among multiple signal-activated phospholipases. Trends Biochem Sci. 1992 Oct;17(10):393–399. doi: 10.1016/0968-0004(92)90007-v. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ohanian J., Ollerenshaw J., Collins P., Heagerty A. Agonist-induced production of 1,2-diacylglycerol and phosphatidic acid in intact resistance arteries. Evidence that accumulation of diacylglycerol is not a prerequisite for contraction. J Biol Chem. 1990 May 25;265(15):8921–8928. [PubMed] [Google Scholar]
- Park H. Y., Russakovsky V., Ohno S., Gilchrest B. A. The beta isoform of protein kinase C stimulates human melanogenesis by activating tyrosinase in pigment cells. J Biol Chem. 1993 Jun 5;268(16):11742–11749. [PubMed] [Google Scholar]
- Pettitt T. R., Wakelam M. J. Bombesin stimulates distinct time-dependent changes in the sn-1,2-diradylglycerol molecular species profile from Swiss 3T3 fibroblasts as analysed by 3,5-dinitrobenzoyl derivatization and h.p.l.c. separation. Biochem J. 1993 Jan 15;289(Pt 2):487–495. doi: 10.1042/bj2890487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittelkow M. R., Scott R. E. New techniques for the in vitro culture of human skin keratinocytes and perspectives on their use for grafting of patients with extensive burns. Mayo Clin Proc. 1986 Oct;61(10):771–777. doi: 10.1016/s0025-6196(12)64815-0. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Punnonen K., Yuspa S. H. Ultraviolet light irradiation increases cellular diacylglycerol and induces translocation of diacylglycerol kinase in murine keratinocytes. J Invest Dermatol. 1992 Aug;99(2):221–226. doi: 10.1111/1523-1747.ep12650445. [DOI] [PubMed] [Google Scholar]
- Schieven G. L., Kirihara J. M., Gilliland L. K., Uckun F. M., Ledbetter J. A. Ultraviolet radiation rapidly induces tyrosine phosphorylation and calcium signaling in lymphocytes. Mol Biol Cell. 1993 May;4(5):523–530. doi: 10.1091/mbc.4.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søndergaard J., Bisgaard H., Thorsen S. Eicosanoids in skin UV inflammation. Photodermatol. 1985 Dec;2(6):359–366. [PubMed] [Google Scholar]
- Wilkins L., Gilchrest B. A., Szabo G., Weinstein R., Maciag T. The stimulation of normal human melanocyte proliferation in vitro by melanocyte growth factor from bovine brain. J Cell Physiol. 1985 Mar;122(3):350–361. doi: 10.1002/jcp.1041220304. [DOI] [PubMed] [Google Scholar]
- Wille J. J., Jr, Pittelkow M. R., Shipley G. D., Scott R. E. Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyses, growth kinetics, and cell cycle studies. J Cell Physiol. 1984 Oct;121(1):31–44. doi: 10.1002/jcp.1041210106. [DOI] [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]