Abstract
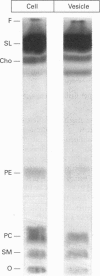
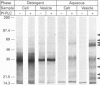
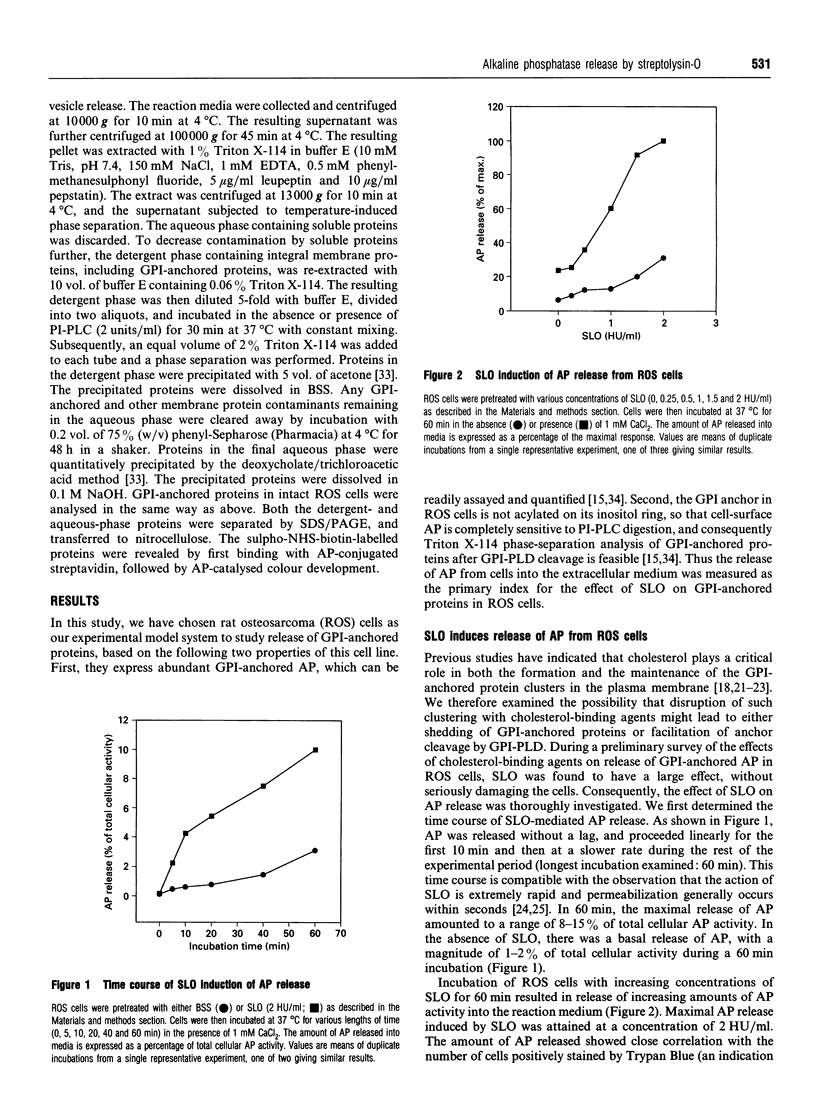
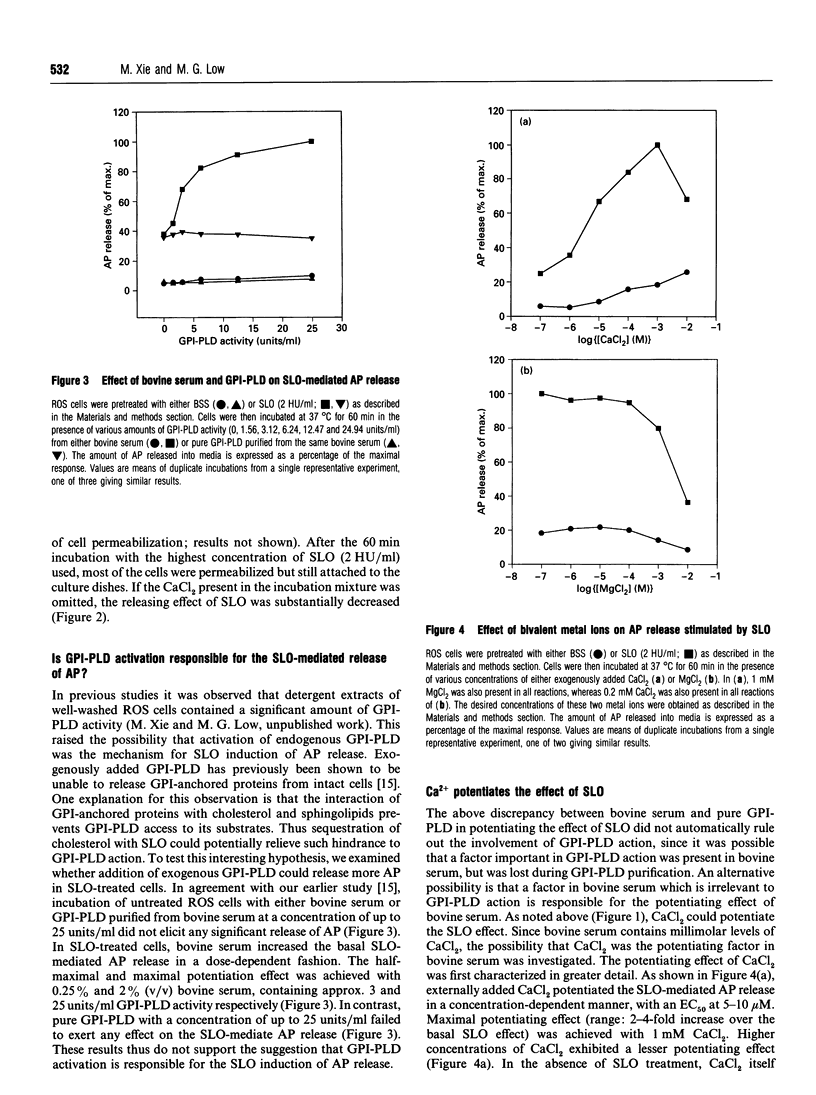
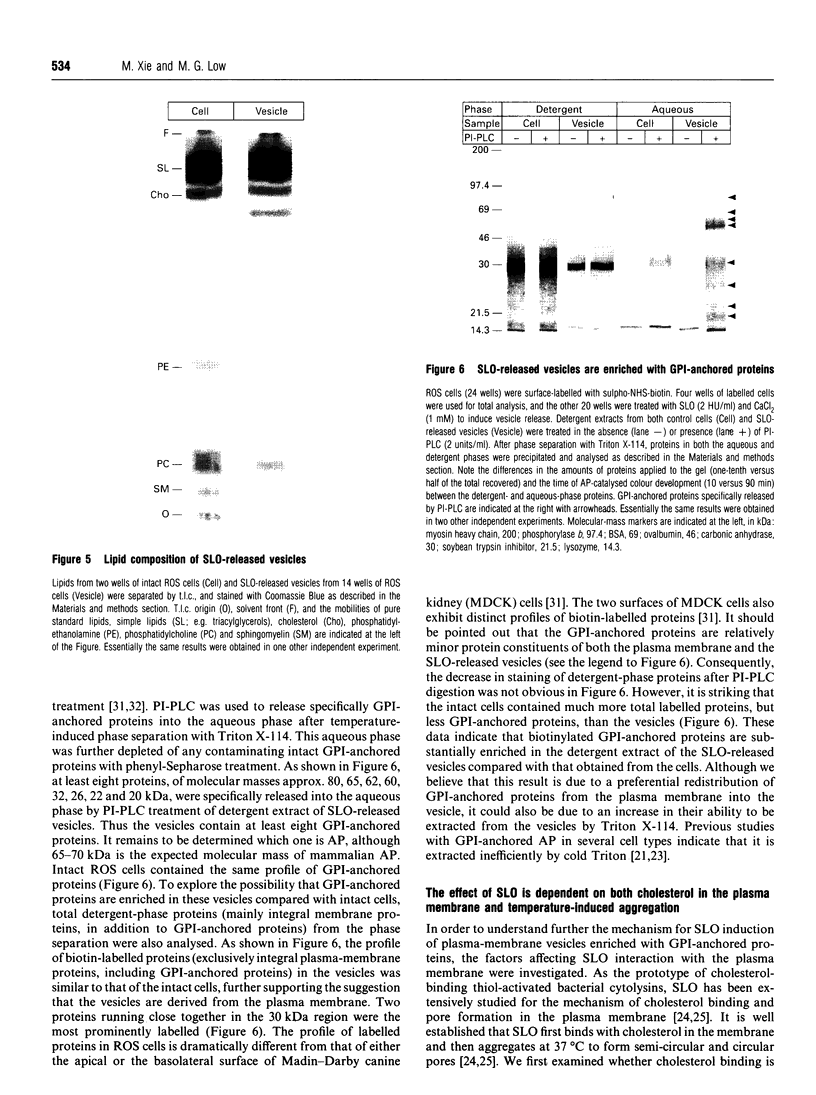
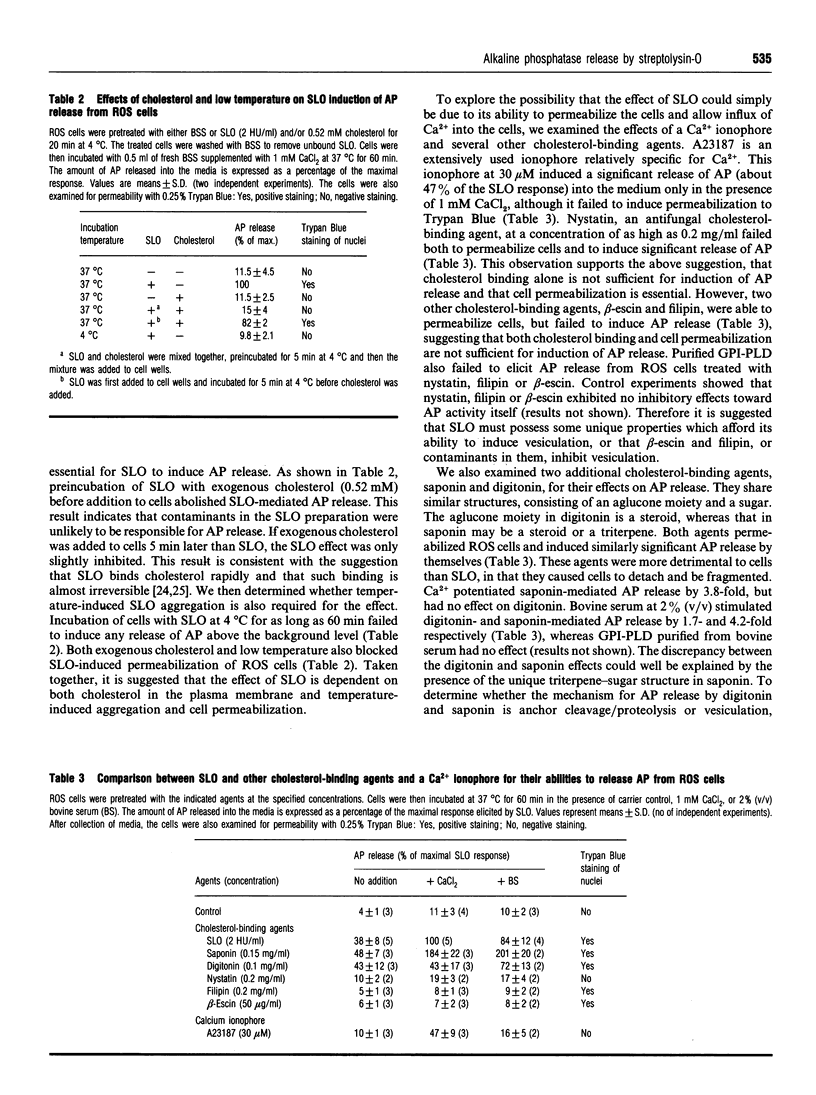
Streptolysin-O (SLO), a cholesterol-binding agent, was used for studies on the release of glycosylphosphatidylinositol (GPI)-anchored alkaline phosphatase (AP) from ROS cells. Treatment of cells with SLO resulted in a time- and concentration-dependent release of AP into the extracellular medium. This release was potentiated by Ca2+ and bovine serum, but not by GPI-specific phospholipase D (GPI-PLD) purified from bovine serum. The released AP distributed to the detergent phase after Triton X-114 phase separation. This result suggested that the released AP contained an intact GPI anchor, and thus both proteolysis and anchor degradation by anchor-specific hydrolases, including GPI-PLD, as the potential mechanisms for SLO-mediated AP release were ruled out. The released AP sedimented at 100,000 g. A substantial amount of lipids was detected in the 100,000 g pellet. Cholesterol and sphingomyelin were enriched in SLO-released material, compared with intact cells. These results were consistent with vesiculation as the mechanism for SLO induction of AP release. Two other cholesterol-binding agents, saponin and digitonin, were also able to release AP, possibly by a similar vesiculation mechanism, whereas others, including nystatin, filipin and beta-escin, failed to elicit any AP release. Eight GPI-anchored proteins were identified in ROS cells, and all were substantially enriched in the vesicles released by SLO. Taken together, these results do not provide any support for the hypothesis that the clustering of GPI-anchored proteins in the plasma membrane is responsible for their resistance to GPI-PLD cleavage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Thomas P. Ca2+-induced biochemical changes in human erythrocytes and their relation to microvesiculation. Biochem J. 1981 Sep 15;198(3):433–440. doi: 10.1042/bj1980433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Thomas P., Limbrick A. R. The isolation and characterization of 60 nm vesicles ('nanovesicles') produced during ionophore A23187-induced budding of human erythrocytes. Biochem J. 1980 Jun 15;188(3):881–887. doi: 10.1042/bj1880881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992 Jan 24;255(5043):410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bergman A. S., Carlsson S. R. Saponin-induced release of cell-surface-anchored Thy-1 by serum glycosylphosphatidylinositol-specific phospholipase D. Biochem J. 1994 Mar 15;298(Pt 3):661–668. doi: 10.1042/bj2980661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Weller U., Walev I., Martin E., Jonas D., Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol. 1993 Sep;182(4):167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brunner G., Metz C. N., Nguyen H., Gabrilove J., Patel S. R., Davitz M. A., Rifkin D. B., Wilson E. L. An endogenous glycosylphosphatidylinositol-specific phospholipase D releases basic fibroblast growth factor-heparan sulfate proteoglycan complexes from human bone marrow cultures. Blood. 1994 Apr 15;83(8):2115–2125. [PubMed] [Google Scholar]
- Bütikofer P., Kuypers F. A., Xu C. M., Chiu D. T., Lubin B. Enrichment of two glycosyl-phosphatidylinositol-anchored proteins, acetylcholinesterase and decay accelerating factor, in vesicles released from human red blood cells. Blood. 1989 Oct;74(5):1481–1485. [PubMed] [Google Scholar]
- Carr J. M., Dvorak A. M., Dvorak H. F. Circulating membrane vesicles in leukemic blood. Cancer Res. 1985 Nov;45(11 Pt 2):5944–5951. [PubMed] [Google Scholar]
- Cerneus D. P., Ueffing E., Posthuma G., Strous G. J., van der Ende A. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. Role of cholesterol. J Biol Chem. 1993 Feb 15;268(5):3150–3155. [PubMed] [Google Scholar]
- Chang W. J., Rothberg K. G., Kamen B. A., Anderson R. G. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992 Jul;118(1):63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hereld D., Shak S., Krakow J., Englund P. T., Nussenzweig V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science. 1987 Oct 2;238(4823):81–84. doi: 10.1126/science.2443973. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hom J., Schenkman S. Purification of a glycosyl-phosphatidylinositol-specific phospholipase D from human plasma. J Biol Chem. 1989 Aug 15;264(23):13760–13764. [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan L. A., Lisanti M. P., Rodriguez-Boulan E., Edidin M. Correctly sorted molecules of a GPI-anchored protein are clustered and immobile when they arrive at the apical surface of MDCK cells. J Cell Biol. 1993 Jan;120(2):353–358. doi: 10.1083/jcb.120.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Li S., Fung W. J., Hulmes J. D., Reik L., Pan Y. C., Low M. G. Purification and characterization of glycosyl-phosphatidylinositol-specific phospholipase D. J Biol Chem. 1990 Oct 15;265(29):17738–17745. [PubMed] [Google Scholar]
- Huang K. S., Li S., Low M. G. Glycosylphosphatidylinositol-specific phospholipase D. Methods Enzymol. 1991;197:567–575. doi: 10.1016/0076-6879(91)97184-z. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Okamoto H., Yamada J., Setaka M., Kwan T. Vesiculation of platelet plasma membranes. Dilauroylglycerophosphocholine-induced shedding of a platelet plasma membrane fraction enriched in acetylcholinesterase activity. Biochim Biophys Acta. 1984 Nov 21;778(1):210–218. doi: 10.1016/0005-2736(84)90464-4. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Sargiacomo M., Graeve L., Saltiel A. R., Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Tang Z. L., Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J Cell Biol. 1993 Nov;123(3):595–604. doi: 10.1083/jcb.123.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Huang K. S. Factors affecting the ability of glycosylphosphatidylinositol-specific phospholipase D to degrade the membrane anchors of cell surface proteins. Biochem J. 1991 Oct 15;279(Pt 2):483–493. doi: 10.1042/bj2790483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Prasad A. R. A phospholipase D specific for the phosphatidylinositol anchor of cell-surface proteins is abundant in plasma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):980–984. doi: 10.1073/pnas.85.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Masella R., Cantafora A., Guidoni L., Luciani A. M., Mariutti G., Rosi A., Viti V. Characterization of vesicles, containing an acylated oligopeptide, released by human colon adenocarcinoma cells. NMR and biochemical studies. FEBS Lett. 1989 Mar 27;246(1-2):25–29. doi: 10.1016/0014-5793(89)80246-7. [DOI] [PubMed] [Google Scholar]
- Mayor S., Rothberg K. G., Maxfield F. R. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994 Jun 24;264(5167):1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- Metz C. N., Brunner G., Choi-Muira N. H., Nguyen H., Gabrilove J., Caras I. W., Altszuler N., Rifkin D. B., Wilson E. L., Davitz M. A. Release of GPI-anchored membrane proteins by a cell-associated GPI-specific phospholipase D. EMBO J. 1994 Apr 1;13(7):1741–1751. doi: 10.1002/j.1460-2075.1994.tb06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz C. N., Thomas P., Davitz M. A. Immunolocalization of a glycosylphosphatidylinositol-specific phospholipase D in mast cells found in normal tissue and neurofibromatosis lesions. Am J Pathol. 1992 Jun;140(6):1275–1281. [PMC free article] [PubMed] [Google Scholar]
- Metz C. N., Zhang Y. Y., Guo Y., Tsang T. C., Kochan J. P., Altszuler N., Davitz M. A. Production of the glycosylphosphatidylinositol-specific phospholipase D by the islets of Langerhans. J Biol Chem. 1991 Sep 25;266(27):17733–17736. [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kamen B. A., Anderson R. G. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990 Dec;111(6 Pt 2):2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann B., Zurbriggen A., Brodbeck U. Distribution of glycosylphosphatidylinositol-specific phospholipase D mRNA in bovine tissue sections. Cell Tissue Res. 1993 Dec;274(3):547–552. doi: 10.1007/BF00314552. [DOI] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Taylor D. D., Black P. H. Shedding of plasma membrane fragments. Neoplastic and developmental importance. Dev Biol (N Y 1985) 1986;3:33–57. doi: 10.1007/978-1-4684-5050-7_3. [DOI] [PubMed] [Google Scholar]
- Wong Y. W., Low M. G. Phospholipase resistance of the glycosyl-phosphatidylinositol membrane anchor on human alkaline phosphatase. Clin Chem. 1992 Dec;38(12):2517–2525. [PubMed] [Google Scholar]
- Xie M. S., Jacobs L. S., Dubyak G. R. Regulation of phospholipase D and primary granule secretion by P2-purinergic- and chemotactic peptide-receptor agonists is induced during granulocytic differentiation of HL-60 cells. J Clin Invest. 1991 Jul;88(1):45–54. doi: 10.1172/JCI115303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Low M. G. Expression and secretion of glycosylphosphatidylinositol-specific phospholipase D by myeloid cell lines. Biochem J. 1994 Feb 1;297(Pt 3):547–554. doi: 10.1042/bj2970547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Sesko A. M., Low M. G. Glycosylphosphatidylinositol-specific phospholipase D is localized in keratinocytes. Am J Physiol. 1993 Oct;265(4 Pt 1):C1156–C1168. doi: 10.1152/ajpcell.1993.265.4.C1156. [DOI] [PubMed] [Google Scholar]
- Ying Y. S., Anderson R. G., Rothberg K. G. Each caveola contains multiple glycosyl-phosphatidylinositol-anchored membrane proteins. Cold Spring Harb Symp Quant Biol. 1992;57:593–604. doi: 10.1101/sqb.1992.057.01.065. [DOI] [PubMed] [Google Scholar]