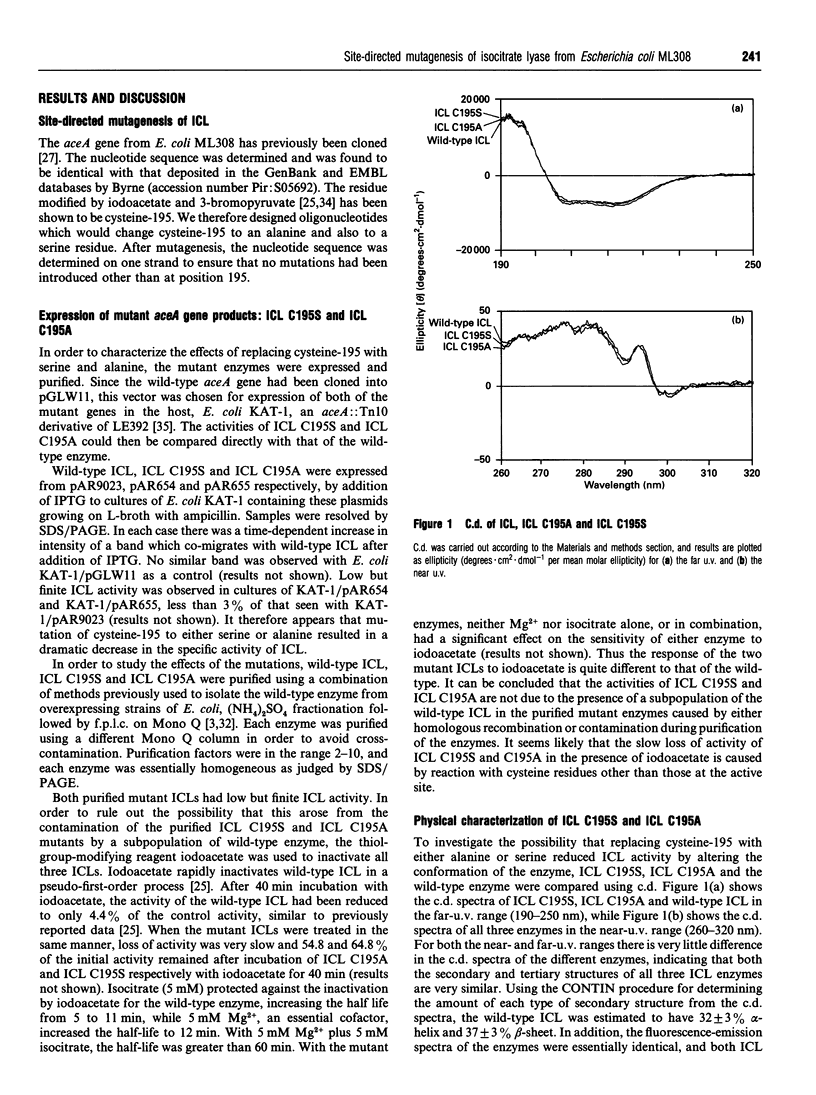
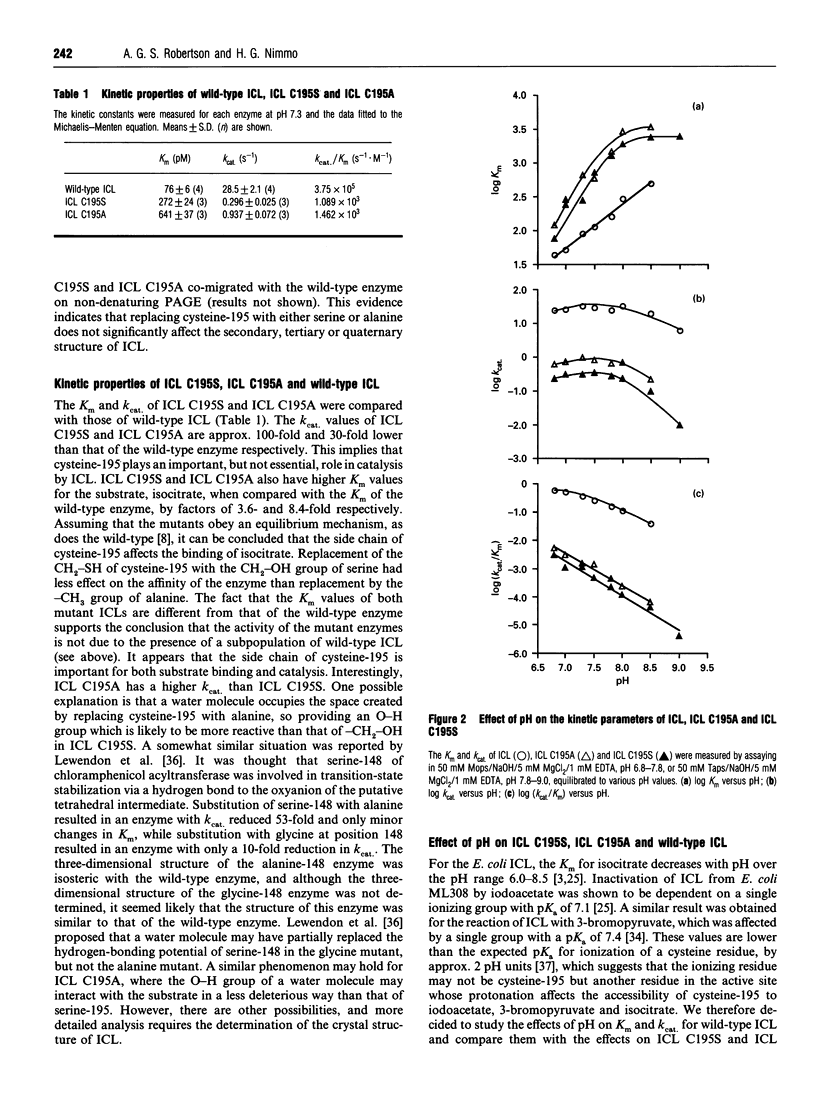
Abstract
Cysteine-195 was previously identified as a probable active site residue in isocitrate lyase (ICL) from Escherichia coli ML308 [Nimmo, Douglas, Kleanthous, Campbell and MacKintosh (1989) Biochem. J. 261, 431-435]. This residue was replaced with serine and alanine residues by site-directed mutagenesis. The mutated genes expressed proteins with low but finite ICL activity, which co-migrated with wild-type ICL on both SDS/ and native PAGE. The mutant proteins were purified and characterized. Fluorimetry and c.d. in both the near- and the far-u.v. regions showed no differences between the mutants and wild-type ICL, indicating that the conformations of the three enzymes were very similar. ICL C195A (Cys-195-->Ala) and C195S (Cys-195-->Ser) showed 8.4-fold and 3.6-fold increases in the Km for isocitrate, while their kcat. values showed 30- and 100-fold decreases respectively. The effect of pH on the kinetic properties of the wild-type and mutant ICLs was investigated. The results showed that the response of the mutant enzymes to pH was simpler than that of the wild-type. For the mutants, ionisation of a group with a pKa of approx. 7.8 affected the Km for isocitrate and kcat.. For the wild-type enzyme, these parameters were affected by the ionization of two or more groups, one of which is presumed to by cysteine-195. The results are consistent with the view that the previously identified group with a pKa of 7.1 whose ionization affects the reaction of ICL by iodoacetate is cysteine-195 itself.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeysinghe S. I., Baker P. J., Rice D. W., Rodgers H. F., Stillman T. J., Ko Y. H., McFadden B. A., Nimmo H. G. Use of chemical modification in the crystallization of isocitrate lyase from Escherichia coli. J Mol Biol. 1991 Jul 5;220(1):13–16. doi: 10.1016/0022-2836(91)90376-h. [DOI] [PubMed] [Google Scholar]
- Atomi H., Ueda M., Hikida M., Hishida T., Teranishi Y., Tanaka A. Peroxisomal isocitrate lyase of the n-alkane-assimilating yeast Candida tropicalis: gene analysis and characterization. J Biochem. 1990 Feb;107(2):262–266. doi: 10.1093/oxfordjournals.jbchem.a123036. [DOI] [PubMed] [Google Scholar]
- Borthwick A. C., Holms W. H., Nimmo H. G. The phosphorylation of Escherichia coli isocitrate dehydrogenase in intact cells. Biochem J. 1984 Sep 15;222(3):797–804. doi: 10.1042/bj2220797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Comai L., Dietrich R. A., Maslyar D. J., Baden C. S., Harada J. J. Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell. 1989 Mar;1(3):293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conder M. J., Ko Y. H., McFadden B. A. Purification of isocitrate lyase from Escherichia coli and watermelon using fast protein liquid chromatography. Prep Biochem. 1988;18(4):431–442. doi: 10.1080/00327488808062542. [DOI] [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameel S., El-Gul T., McFadden B. A. Modification of the active site of isocitrate lyase from watermelon cotyledons. Arch Biochem Biophys. 1985 Jan;236(1):72–81. doi: 10.1016/0003-9861(85)90607-1. [DOI] [PubMed] [Google Scholar]
- Johanson R. A., Hill J. M., McFadden B. A. Isocitrate lyase from Neurospora crassa. II. Composition, quaternary structure, C-terminus, and active-site modification. Biochim Biophys Acta. 1974 Oct 17;364(2):341–352. doi: 10.1016/0005-2744(74)90019-9. [DOI] [PubMed] [Google Scholar]
- Khan F. R., McFadden B. A. Isocitrate lyase from flax : terminal residues, composition, active site, and catalysis. Plant Physiol. 1982 Oct;70(4):943–948. doi: 10.1104/pp.70.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y. H., Cremo C. R., McFadden B. A. Vanadate-dependent photomodification of serine 319 and 321 in the active site of isocitrate lyase from Escherichia coli. J Biol Chem. 1992 Jan 5;267(1):91–95. [PubMed] [Google Scholar]
- Ko Y. H., McFadden B. A. Alkylation of isocitrate lyase from Escherichia coli by 3-bromopyruvate. Arch Biochem Biophys. 1990 May 1;278(2):373–380. doi: 10.1016/0003-9861(90)90273-2. [DOI] [PubMed] [Google Scholar]
- Ko Y. H., Vanni P., Munske G. R., McFadden B. A. Substrate-decreased modification by diethyl pyrocarbonate of two histidines in isocitrate lyase from Escherichia coli. Biochemistry. 1991 Jul 30;30(30):7451–7456. doi: 10.1021/bi00244a012. [DOI] [PubMed] [Google Scholar]
- Laporte D. C., Stueland C. S., Ikeda T. P. Isocitrate dehydrogenase kinase/phosphatase. Biochimie. 1989 Sep-Oct;71(9-10):1051–1057. doi: 10.1016/0300-9084(89)90110-7. [DOI] [PubMed] [Google Scholar]
- Lewendon A., Murray I. A., Shaw W. V., Gibbs M. R., Leslie A. G. Evidence for transition-state stabilization by serine-148 in the catalytic mechanism of chloramphenicol acetyltransferase. Biochemistry. 1990 Feb 27;29(8):2075–2080. doi: 10.1021/bi00460a016. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Nimmo H. G. Purification and regulatory properties of isocitrate lyase from Escherichia coli ML308. Biochem J. 1988 Feb 15;250(1):25–31. doi: 10.1042/bj2500025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra O. P., Dwivedi U. N., Singh J., Srivastava P. K. Chemical reaction mechanism and active-site groups of isocitrate lyase. Indian J Biochem Biophys. 1987 Oct;24(5):suppl–62. [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):173–180. doi: 10.1128/jb.149.1.173-180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., McFadden B. A. Isolation, hyperexpression, and sequencing of the aceA gene encoding isocitrate lyase in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4528–4536. doi: 10.1128/jb.170.10.4528-4536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Rao G. R., Cohen A. L., Roche T. E. Isocitrate lyase from Pseudomonas indigofera. V. Subunits and terminal residues and the relation to catalytic activity. Biochemistry. 1968 Oct;7(10):3574–3582. doi: 10.1021/bi00850a035. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Douglas F., Kleanthous C., Campbell D. G., MacKintosh C. Identification of a cysteine residue at the active site of Escherichia coli isocitrate lyase. Biochem J. 1989 Jul 15;261(2):431–435. doi: 10.1042/bj2610431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieul C., Bleicher F., Duclos B., Cortay J. C., Cozzone A. J. Nucleotide sequence of the aceA gene coding for isocitrate lyase in Escherichia coli. Nucleic Acids Res. 1988 Jun 24;16(12):5689–5689. doi: 10.1093/nar/16.12.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche T. E., McFadden B. A. Active site modification of isocitrate lyase. Biochem Biophys Res Commun. 1969 Oct 8;37(2):239–246. doi: 10.1016/0006-291x(69)90725-6. [DOI] [PubMed] [Google Scholar]
- Roche T. E., McFadden B. A., Williams J. O. Modification of the active site of isocitrate lyase from Pseudomonas indigofera. Arch Biochem Biophys. 1971 Nov;147(1):192–200. doi: 10.1016/0003-9861(71)90327-4. [DOI] [PubMed] [Google Scholar]
- Rua J., Robertson A. G., Nimmo H. G. Identification of the histidine residue in Escherichia coli isocitrate lyase that reacts with diethylpyrocarbonate. Biochim Biophys Acta. 1992 Jul 31;1122(2):212–218. doi: 10.1016/0167-4838(92)90326-9. [DOI] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley R. B., Choe S. M., Trelease R. N. Characterization of a cDNA clone encoding the complete amino acid sequence of cotton isocitrate lyase. Biochim Biophys Acta. 1990 Jun 21;1049(2):223–226. doi: 10.1016/0167-4781(90)90045-4. [DOI] [PubMed] [Google Scholar]
- Vanni P., Giachetti E., Pinzauti G., McFadden B. A. Comparative structure, function and regulation of isocitrate lyase, an important assimilatory enzyme. Comp Biochem Physiol B. 1990;95(3):431–458. doi: 10.1016/0305-0491(90)90002-b. [DOI] [PubMed] [Google Scholar]
- el-Mansi E. M., MacKintosh C., Duncan K., Holms W. H., Nimmo H. G. Molecular cloning and over-expression of the glyoxylate bypass operon from Escherichia coli ML308. Biochem J. 1987 Mar 15;242(3):661–665. doi: 10.1042/bj2420661. [DOI] [PMC free article] [PubMed] [Google Scholar]


