Abstract
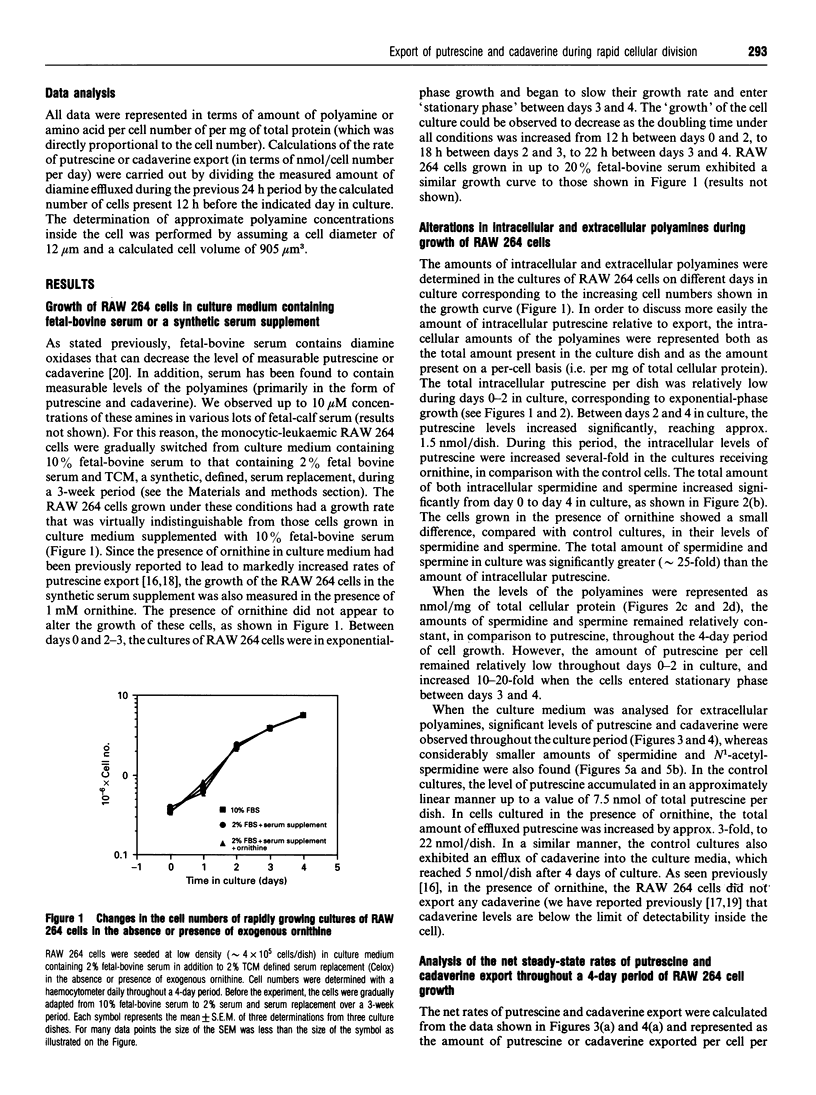
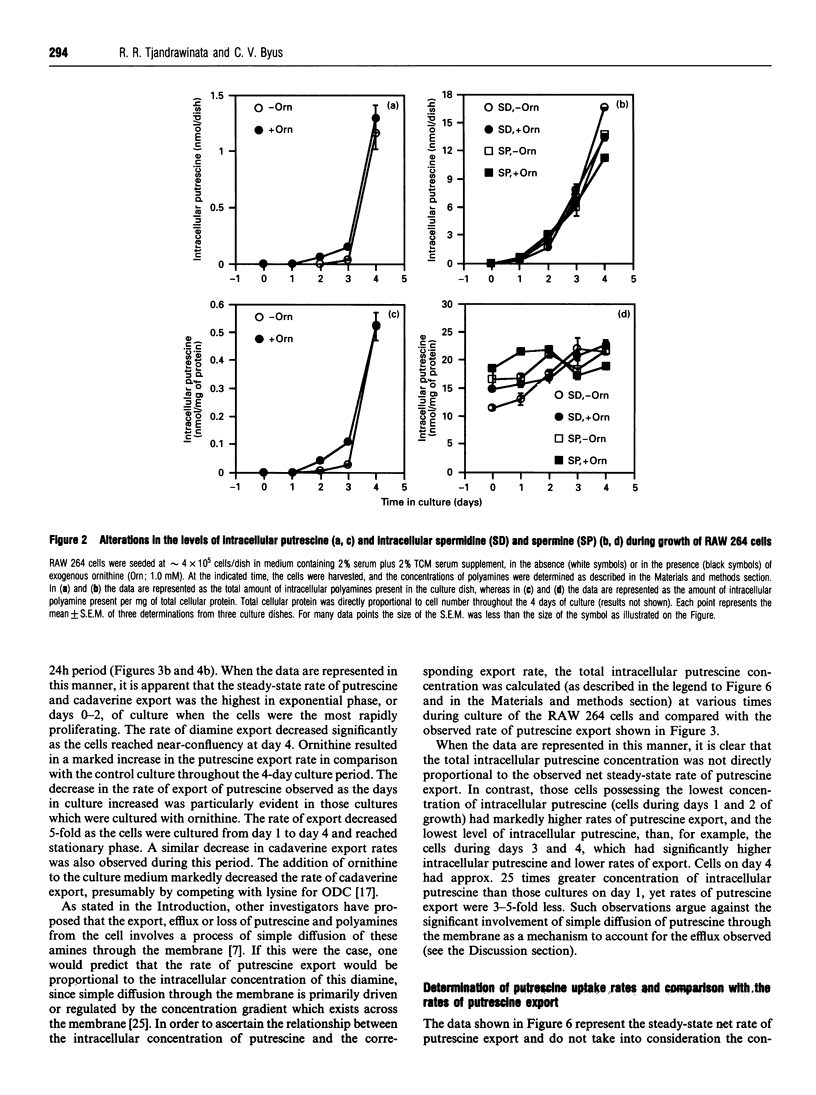
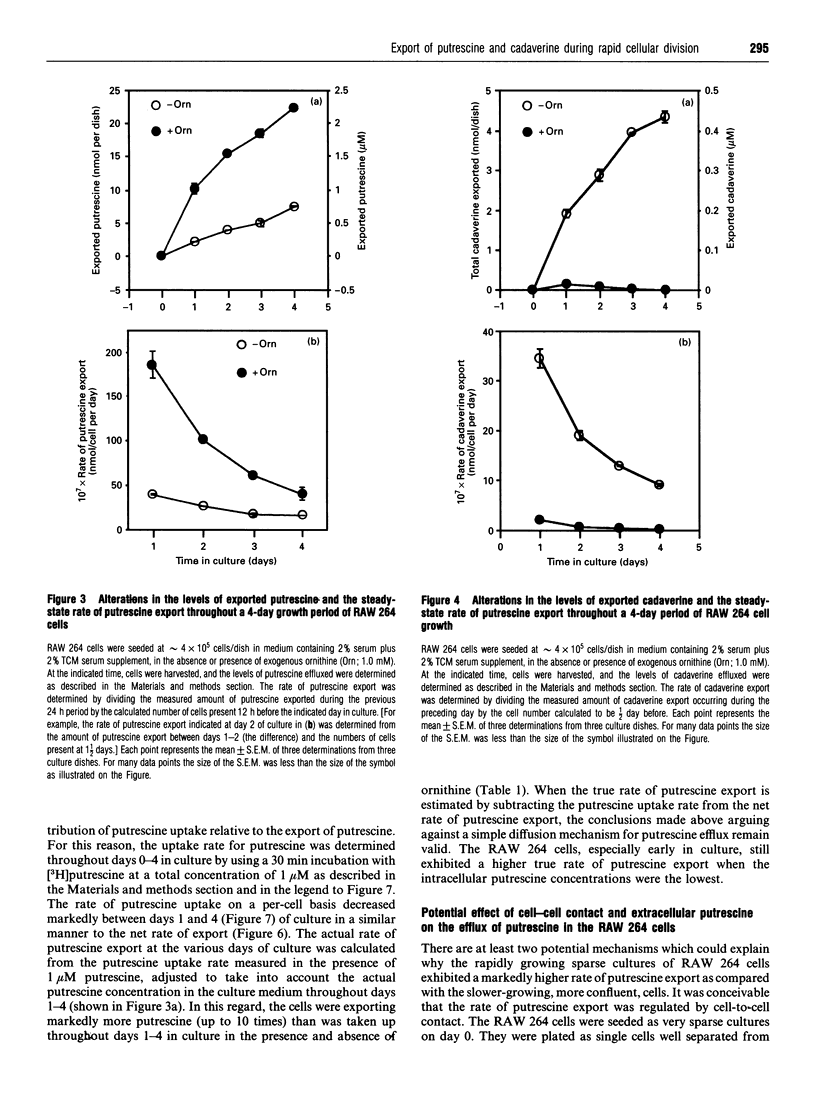
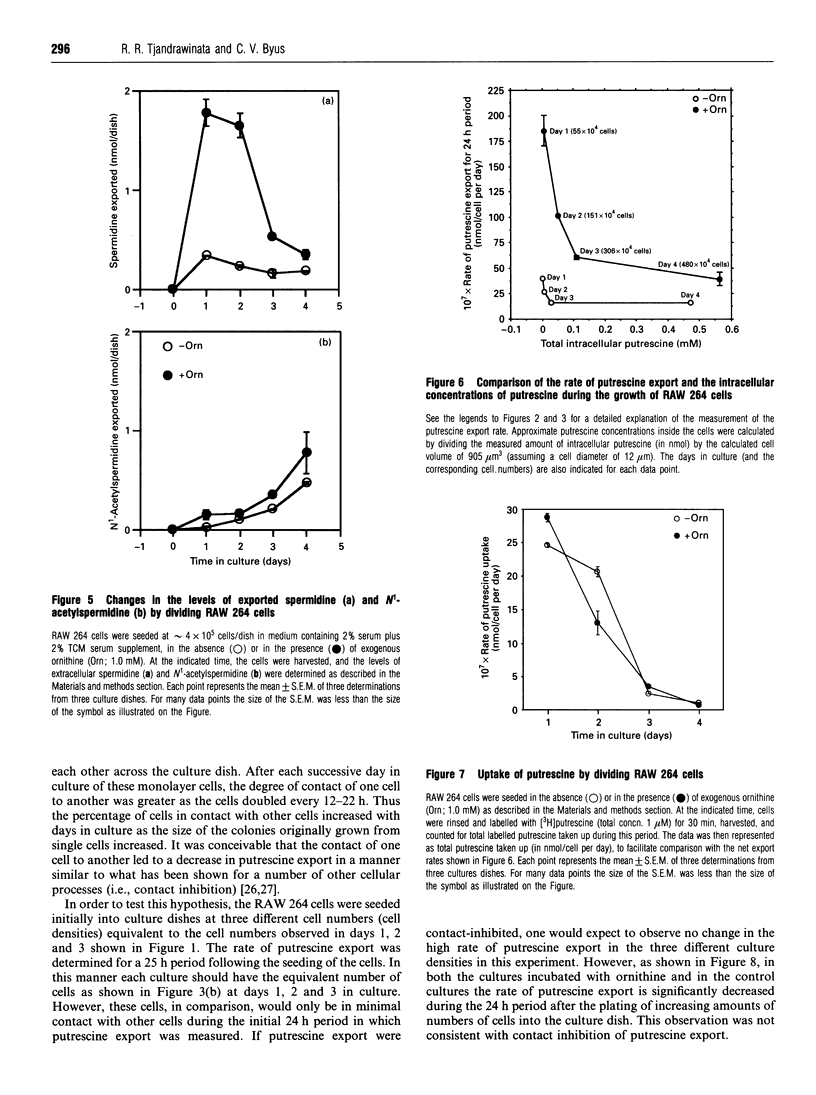
Cultures of the macrophage-like RAW 264 cells were adapted to divide normally in a synthetic serum-supplemented culture medium lacking any polyamines and diamine oxidase activity. These rapidly dividing cells actively effluxed large amounts of putrescine and cadaverine, compared with the intracellular levels, into the culture medium. The efflux of putrescine was stimulated by the amino acid ornithine, whereas efflux of cadaverine was inhibited. Relatively low levels of spermidine and N1-acetyl-spermidine, compared with those of exported putrescine, were observed to accumulate in the culture medium. A careful analysis of the changes in the intracellular concentration of putrescine relative to the steady-state net rate of putrescine export, as the doubling time of the cultures increased from 16 h to 22 h, indicated that an inverse relationship existed between these two parameters. As the intracellular putrescine concentrations increased, the net rate of putrescine export decreased markedly. Determination of the rate of putrescine uptake indicated that putrescine uptake also decreased significantly as the cultures neared confluency, and at no time during the growth of the culture did the rate of putrescine uptake approximate to the high rate of putrescine efflux. The decrease in the putrescine export rate seen as the cells grew toward confluency was determined to be primarily due to the inhibitory effect of the effluxed putrescine in the medium (Ki = 2 microM), and not to contact inhibition. The data suggested that the efflux of putrescine and cadaverine is not mediated to a significant degree by a process involving simple diffusion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Casero R. A., Jr, Pegg A. E. Spermidine/spermine N1-acetyltransferase--the turning point in polyamine metabolism. FASEB J. 1993 May;7(8):653–661. [PubMed] [Google Scholar]
- Davis R. H., Morris D. R., Coffino P. Sequestered end products and enzyme regulation: the case of ornithine decarboxylase. Microbiol Rev. 1992 Jun;56(2):280–290. doi: 10.1128/mr.56.2.280-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Ristow J. L. Uptake, intracellular binding, and excretion of polyamines during growth of Neurospora crassa. Arch Biochem Biophys. 1989 Jun;271(2):315–322. doi: 10.1016/0003-9861(89)90281-6. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Pitot H. C. Polyamine degradation in foetal and adult bovine serum. Biochem J. 1982 Mar 15;202(3):603–611. doi: 10.1042/bj2020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R. S., Gonzalez G. G., Hawel L., 3rd, Byus C. V. An ion-exchange chromatography procedure for the isolation and concentration of basic amino acids and polyamines from complex biological samples prior to high-performance liquid chromatography. Anal Biochem. 1991 Nov 15;199(1):86–92. doi: 10.1016/0003-2697(91)90273-v. [DOI] [PubMed] [Google Scholar]
- Hawel L., 3rd, Tjandrawinata R. R., Byus C. V. Selective putrescine export is regulated by insulin and ornithine in Reuber H35 hepatoma cells. Biochim Biophys Acta. 1994 May 26;1222(1):15–26. doi: 10.1016/0167-4889(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Heby O., Persson L. Molecular genetics of polyamine synthesis in eukaryotic cells. Trends Biochem Sci. 1990 Apr;15(4):153–158. doi: 10.1016/0968-0004(90)90216-x. [DOI] [PubMed] [Google Scholar]
- Hyvönen T. Excretion of acetylated and free polyamines by polyamine depleted Chinese hamster ovary cells. Int J Biochem. 1989;21(3):313–316. doi: 10.1016/0020-711x(89)90189-4. [DOI] [PubMed] [Google Scholar]
- Kabra P. M., Lee H. K., Lubich W. P., Marton L. J. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr. 1986 Jul 11;380(1):19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- Kameji T., Hayashi S., Hoshino K., Kakinuma Y., Igarashi K. Multiple regulation of ornithine decarboxylase in enzyme-overproducing cells. Biochem J. 1993 Jan 15;289(Pt 2):581–586. doi: 10.1042/bj2890581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K., Hosokawa N., Furuchi T., Kobayashi H., Sasakawa C., Yoshikawa M., Igarashi K. Isolation of polyamine transport-deficient mutants of Escherichia coli and cloning of the genes for polyamine transport proteins. J Biol Chem. 1990 Dec 5;265(34):20893–20897. [PubMed] [Google Scholar]
- Kashiwagi K., Yamaguchi Y., Sakai Y., Kobayashi H., Igarashi K. Identification of the polyamine-induced protein as a periplasmic oligopeptide binding protein. J Biol Chem. 1990 May 25;265(15):8387–8391. [PubMed] [Google Scholar]
- Lehnert M., Dalton W. S., Roe D., Emerson S., Salmon S. E. Synergistic inhibition by verapamil and quinine of P-glycoprotein-mediated multidrug resistance in a human myeloma cell line model. Blood. 1991 Jan 15;77(2):348–354. [PubMed] [Google Scholar]
- Lum B. L., Gosland M. P., Kaubisch S., Sikic B. I. Molecular targets in oncology: implications of the multidrug resistance gene. Pharmacotherapy. 1993 Mar-Apr;13(2):88–109. [PubMed] [Google Scholar]
- Pegg A. E., Pakala R., Bergeron R. J. Induction of spermidine/spermine N1-acetyltransferase activity in Chinese-hamster ovary cells by N1N11-bis(ethyl)norspermine (corrected) and related compounds. Biochem J. 1990 Apr 15;267(2):331–338. doi: 10.1042/bj2670331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Wechter R., Pakala R., Bergeron R. J. Effect of N1,N12-bis(ethyl)spermine and related compounds on growth and polyamine acetylation, content, and excretion in human colon tumor cells. J Biol Chem. 1989 Jul 15;264(20):11744–11749. [PubMed] [Google Scholar]
- Porter C. W., Ganis B., Libby P. R., Bergeron R. J. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991 Jul 15;51(14):3715–3720. [PubMed] [Google Scholar]
- Seiler N., Dezeure F. Polyamine transport in mammalian cells. Int J Biochem. 1990;22(3):211–218. doi: 10.1016/0020-711x(90)90332-w. [DOI] [PubMed] [Google Scholar]
- Seiler N. Functions of polyamine acetylation. Can J Physiol Pharmacol. 1987 Oct;65(10):2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tjandrawinata R. R., Hawel L., 3rd, Byus C. V. Regulation of putrescine export in lipopolysaccharide or IFN-gamma-activated murine monocytic-leukemic RAW 264 cells. J Immunol. 1994 Mar 15;152(6):3039–3052. [PubMed] [Google Scholar]
- Wallace H. M., Coleman C. S. Changes in polyamine acetylation in human cancer cells. Biochem Soc Trans. 1990 Dec;18(6):1091–1094. doi: 10.1042/bst0181091. [DOI] [PubMed] [Google Scholar]
- Wallace H. M., Keir H. M. Factors affecting polyamine excretion from mammalian cells in culture. Inhibitors of polyamine biosynthesis. FEBS Lett. 1986 Jan 1;194(1):60–63. doi: 10.1016/0014-5793(86)80051-5. [DOI] [PubMed] [Google Scholar]
- Wallace H. M., Keir H. M. Uptake and excretion of polyamines from baby hamster kidney cells (BHK-21/C13). The effect of serum on confluent cell cultures. Biochim Biophys Acta. 1981 Aug 5;676(1):25–30. doi: 10.1016/0304-4165(81)90005-2. [DOI] [PubMed] [Google Scholar]
- Wieser R. J., Renauer D., Schäfer A., Heck R., Engel R., Schütz S., Oesch F. Growth control in mammalian cells by cell-cell contacts. Environ Health Perspect. 1990 Aug;88:251–253. doi: 10.1289/ehp.9088251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Romano C., Dichter M. A., Molinoff P. B. Modulation of the NMDA receptor by polyamines. Life Sci. 1991;48(6):469–498. doi: 10.1016/0024-3205(91)90463-l. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J. Phenotypic transformation of normal rat kidney cells: a model for studying cellular alterations in oncogenesis. Crit Rev Oncog. 1991;2(4):311–333. [PubMed] [Google Scholar]


