Abstract
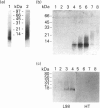
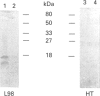
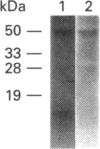
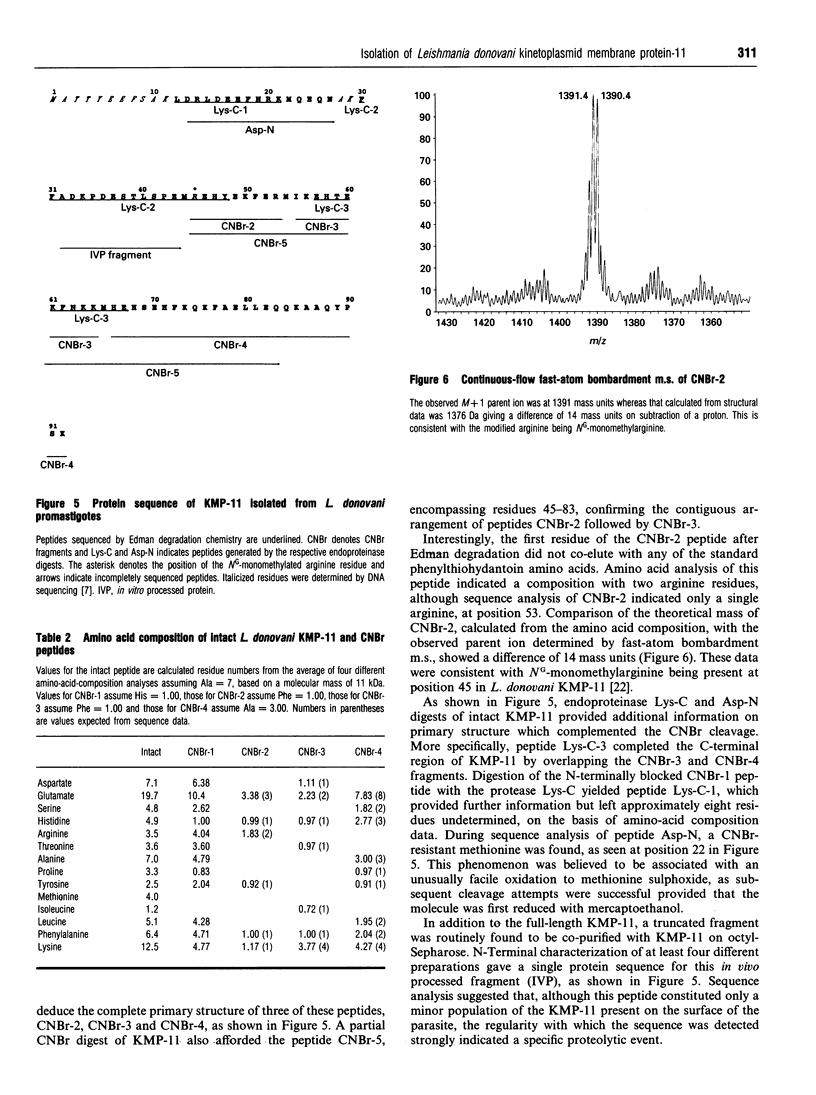
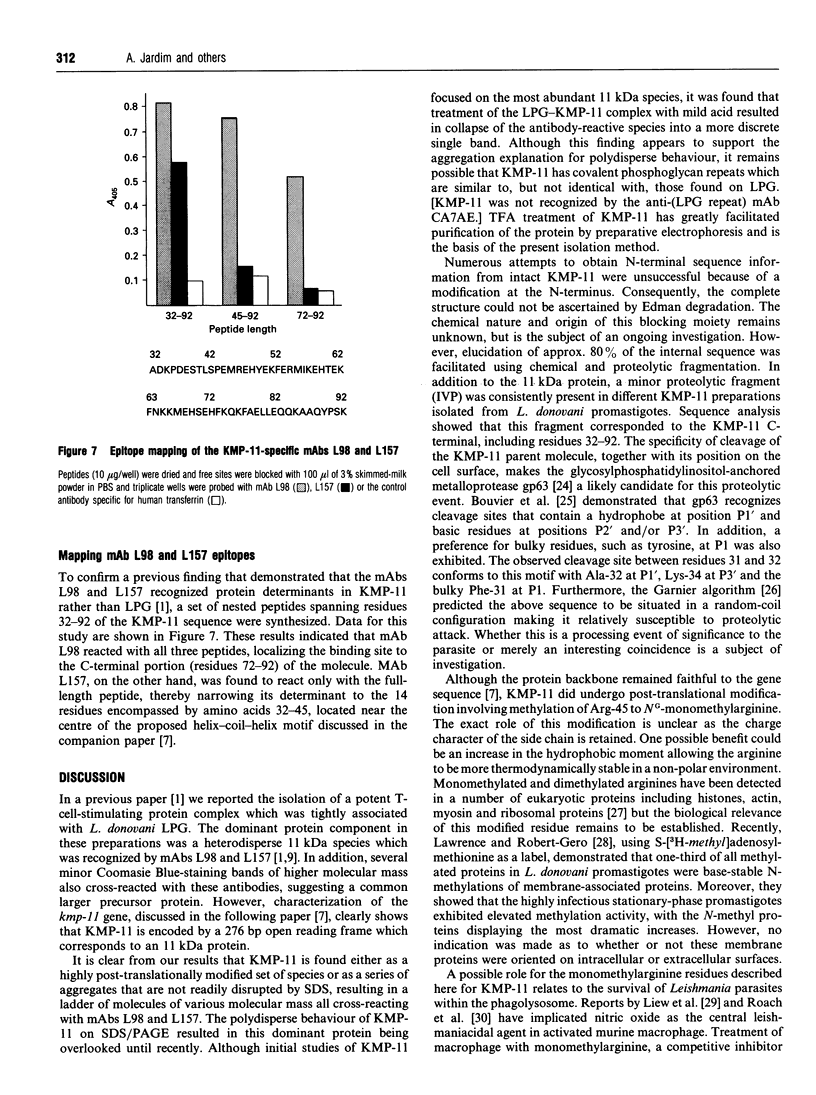
A novel membrane molecule, previously observed to be co-isolated with lipophosphoglycan and called lipophosphoglycan-associated protein, has been detected in Leishmania donovani promastigotes and amastigotes. This kinetoplastid membrane protein (KMP-11) has been purified by preparative SDS/PAGE after organic solvent extraction of promastigote membranes. Isoelectric-focusing experiments indicated that this was an acidic protein with an isoelectric point of 4.8. Immunoblot analysis of subcellular fractions, together with 125I-labelling experiments, showed this molecule to be associated with the promastigote cell surface membrane. KMP-11 was expressed at a copy number similar to that of lipophosphoglycan (1 x 10(6)-2 x 10(6) molecules per cell), making this glycoprotein one of the major features on the parasite cell surface. The primary structure, less a blocked N-terminal region, was determined by automated Edman degradation of peptides derived from CNBr or enzymic fragmentation. Several post-translational modifications were also found during these studies, including an O-linked oligosaccharide and an NG-monomethylarginine functionality which was verified by m.s. Finally, a set of sequential synthetic peptides was made based on the established partial sequence allowing structural determination of two distinct antibody-binding sites for the monoclonal antibodies L98 and L157.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Bouvier J., Schneider P., Etges R., Bordier C. Peptide substrate specificity of the membrane-bound metalloprotease of Leishmania. Biochemistry. 1990 Oct 30;29(43):10113–10119. doi: 10.1021/bi00495a015. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Roberts M. B., Blackwell J. M. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984 Oct;53(2):345–355. [PMC free article] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is a protease. J Biol Chem. 1986 Jul 15;261(20):9098–9101. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Dwyer D. M. Leishmania donovani: surface membrane acid phosphatase activity of promastigotes. Exp Parasitol. 1981 Aug;52(1):117–128. doi: 10.1016/0014-4894(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Jarvis H. M., Mitchell G. F. Leishmania major: identification of stage-specific antigens and antigens shared by promastigotes and amastigotes. Parasite Immunol. 1984 May;6(3):223–233. doi: 10.1111/j.1365-3024.1984.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Shor R., Trau H., Passwell J. H. Parasite antigens recognized by patients with cutaneous leishmaniasis. Clin Exp Immunol. 1990 Apr;80(1):77–82. doi: 10.1111/j.1365-2249.1990.tb06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardim A., Hanson S., Ullman B., McCubbin W. D., Kay C. M., Olafson R. W. Cloning and structure-function analysis of the Leishmania donovani kinetoplastid membrane protein-11. Biochem J. 1995 Jan 1;305(Pt 1):315–320. doi: 10.1042/bj3050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardim A., Tolson D. L., Turco S. J., Pearson T. W., Olafson R. W. The Leishmania donovani lipophosphoglycan T lymphocyte-reactive component is a tightly associated protein complex. J Immunol. 1991 Nov 15;147(10):3538–3544. [PubMed] [Google Scholar]
- King D. L., Chang Y. D., Turco S. J. Cell surface lipophosphoglycan of Leishmania donovani. Mol Biochem Parasitol. 1987 May;24(1):47–53. doi: 10.1016/0166-6851(87)90114-9. [DOI] [PubMed] [Google Scholar]
- Kobata A., Yamashita K., Takasaki S. BioGel P-4 column chromatography of oligosaccharides: effective size of oligosaccharides expressed in glucose units. Methods Enzymol. 1987;138:84–94. doi: 10.1016/0076-6879(87)38008-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence F., Robert-Gero M. Distribution of macromolecular methylations in promastigotes of Leishmania donovani and impact of sinefungin. J Eukaryot Microbiol. 1993 Sep-Oct;40(5):581–589. doi: 10.1111/j.1550-7408.1993.tb06111.x. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–4310. [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça S. C., Russell D. G., Coutinho S. G. Analysis of the human T cell responsiveness to purified antigens of Leishmania: lipophosphoglycan (LPG) and glycoprotein 63 (gp 63). Clin Exp Immunol. 1991 Mar;83(3):472–478. doi: 10.1111/j.1365-2249.1991.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Paik W. K. A simple analysis of methylated proteins. Methods Enzymol. 1984;106:265–268. doi: 10.1016/0076-6879(84)06026-2. [DOI] [PubMed] [Google Scholar]
- Roach T. I., Kiderlen A. F., Blackwell J. M. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991 Nov;59(11):3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Tolson D. L., Turco S. J., Beecroft R. P., Pearson T. W. The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Mol Biochem Parasitol. 1989 Jun 15;35(2):109–118. doi: 10.1016/0166-6851(89)90113-8. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Wilkerson M. A., Clawson D. R. Expression of an unusual acidic glycoconjugate in Leishmania donovani. J Biol Chem. 1984 Mar 25;259(6):3883–3889. [PubMed] [Google Scholar]