Abstract
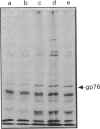
The action of non-detergent sulphobetaines (NDSBs) as new mild agents for protein purification is described. The solubilization effects of non-detergent sulphobetaines are shown in different examples; all obtained under non-denaturing conditions: (1) microsomal proteins extraction; (2) recovery after dialysis of nuclear proteins; (3) reduction of precipitation in isoelectric focusing experiments under non-denaturing conditions; and (4) purification of a membrane-bound serine protease from Plasmodium falciparum involved in erythrocyte invasion by malaria merozoites. The absence of a significant denaturation effect induced by NDSBs is demonstrated by tests on beta-galactosidase and alkaline phosphatase. A simple NDSB synthesis and some possible explanations of the action of NDSBs are also presented.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun Breton C., Pereira da Silva L. H. Malaria proteases and red blood cell invasion. Parasitol Today. 1993 Mar;9(3):92–96. doi: 10.1016/0169-4758(93)90212-x. [DOI] [PubMed] [Google Scholar]
- Braun-Breton C., Jendoubi M., Brunet E., Perrin L., Scaife J., Pereira da Silva L. In vivo time course of synthesis and processing of major schizont membrane polypeptides in Plasmodium falciparum. Mol Biochem Parasitol. 1986 Jul;20(1):33–43. doi: 10.1016/0166-6851(86)90140-4. [DOI] [PubMed] [Google Scholar]
- Braun-Breton C., Pereira da Silva L. Activation of a Plasmodium falciparum protease correlated with merozoite maturation and erythrocyte invasion. Biol Cell. 1988;64(2):223–231. doi: 10.1016/0248-4900(88)90081-0. [DOI] [PubMed] [Google Scholar]
- Breton C. B., Blisnick T., Jouin H., Barale J. C., Rabilloud T., Langsley G., Pereira da Silva L. H. Plasmodium chabaudi p68 serine protease activity required for merozoite entry into mouse erythrocytes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9647–9651. doi: 10.1073/pnas.89.20.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Görg A., Postel W., Günther S. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 1988 Sep;9(9):531–546. doi: 10.1002/elps.1150090913. [DOI] [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland L. M., Nebert D. W., Chrambach A. Electrofocusing of integral membrane proteins in mixtures of zwitterionic and nonionic detergents. Anal Biochem. 1979 May;95(1):201–208. doi: 10.1016/0003-2697(79)90206-9. [DOI] [PubMed] [Google Scholar]
- Hughes G. J., Frutiger S., Paquet N., Ravier F., Pasquali C., Sanchez J. C., James R., Tissot J. D., Bjellqvist B., Hochstrasser D. F. Plasma protein map: an update by microsequencing. Electrophoresis. 1992 Sep-Oct;13(9-10):707–714. doi: 10.1002/elps.11501301150. [DOI] [PubMed] [Google Scholar]
- Matuo Y., Matsui S., Nishi N., Wada F., Sandberg A. A. Quantitative solubilization of nonhistone chromosomal proteins without denaturation using zwitterionic detergents. Anal Biochem. 1985 Nov 1;150(2):337–344. doi: 10.1016/0003-2697(85)90520-2. [DOI] [PubMed] [Google Scholar]
- Navarrete R., Serrano R. Solubilization of yeast plasma membranes and mitochondria by different types of non-denaturing detergents. Biochim Biophys Acta. 1983 Mar 9;728(3):403–408. doi: 10.1016/0005-2736(83)90512-6. [DOI] [PubMed] [Google Scholar]
- Neugebauer J. M. Detergents: an overview. Methods Enzymol. 1990;182:239–253. doi: 10.1016/0076-6879(90)82020-3. [DOI] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Rabilloud T., Gianazza E., Cattò N., Righetti P. G. Amidosulfobetaines, a family of detergents with improved solubilization properties: application for isoelectric focusing under denaturing conditions. Anal Biochem. 1990 Feb 15;185(1):94–102. doi: 10.1016/0003-2697(90)90261-7. [DOI] [PubMed] [Google Scholar]
- Rabilloud T., Vuillard L., Gilly C., Lawrence J. J. Silver-staining of proteins in polyacrylamide gels: a general overview. Cell Mol Biol (Noisy-le-grand) 1994 Feb;40(1):57–75. [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Zulauf M., Fürstenberger U., Grabo M., Jäggi P., Regenass M., Rosenbusch J. P. Critical micellar concentrations of detergents. Methods Enzymol. 1989;172:528–538. doi: 10.1016/s0076-6879(89)72032-2. [DOI] [PubMed] [Google Scholar]