Abstract
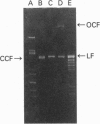
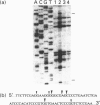
A new exocytoplasmic, nutritionally controlled endodeoxyribonuclease (EC 3.1.21.-) was purified to homogeneity from Streptomyces antibioticus. The enzyme showed an apparent molecular mass of 29 kDa (being active in the monomeric form) and a pI of approximately 7.8. The nuclease hydrolysed endonucleolytically double-stranded circular and linear DNA. The enzyme makes nicks in one strand of the DNA in G-rich regions, leaving either 5' or 3' short, single-stranded overhangs with 3'-hydroxy and 5'-phosphate termini. Breaks in the DNA occur when two nicks in opposite strands are close together. The enzyme had an optimum pH of 7.5 and an absolute requirement for bivalent cations and > or = 100 mM NaCl in the reaction buffer. Activity was greatly diminished in the presence of phosphate, Hg2+ or iodoacetate and was stimulated by dimethyl sulphoxide. Single-stranded DNA was a much poorer substrate than double-stranded DNA. The nuclease hydrolyses sequences of three or preferably more (dG).(dC) tracts in the DNA. The initial specificity shifts to other sequences (including sequences shorter than those initially hydrolysed) during the course of the reaction, giving the changing pattern of bands observed in agarose gels. 5-Methylcytosine-hemimethylated DNA is not hydrolysed by the nuclease. The properties of this novel enzyme suggest a relationship with class II restriction endonucleases and also with some eukaryotic nucleases.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aparicio J. F., De los Reyes-Gavilán C. G., Barbés C., Hardisson C., Sánchez J. A non-specific deoxyribonuclease with restriction function in Streptomyces glaucescens. J Gen Microbiol. 1988 Aug;134(8):2345–2351. doi: 10.1099/00221287-134-8-2345. [DOI] [PubMed] [Google Scholar]
- Aparicio J. F., Freije J. M., Lopez-Otin C., Cal S., Sanchez J. A Streptomyces glaucescens endodeoxyribonuclease which shows a strong preference for CC dinucleotide. Eur J Biochem. 1992 Apr 15;205(2):695–699. doi: 10.1111/j.1432-1033.1992.tb16831.x. [DOI] [PubMed] [Google Scholar]
- Aparicio J. F., Hardisson C., Sánchez J. Purification and characterization of a nutritionally controlled endodeoxyribonuclease from Streptomyces glaucescens. Biochem J. 1992 Jan 1;281(Pt 1):231–237. doi: 10.1042/bj2810231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange as an assay for class II restriction endonucleases. Methods Enzymol. 1980;65(1):28–36. doi: 10.1016/s0076-6879(80)65007-1. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Smith M. A general method for defining restriction enzyme cleavage and recognition sites. Methods Enzymol. 1980;65(1):391–404. doi: 10.1016/s0076-6879(80)65050-2. [DOI] [PubMed] [Google Scholar]
- Burton W. G., Roberts R. J., Myers P. A., Sager R. A site-specific single-strand endonuclease from the eukaryote Chlamydomonas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2687–2691. doi: 10.1073/pnas.74.7.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Cummings O. W., King T. C., Holden J. A., Low R. L. Purification and characterization of the potent endonuclease in extracts of bovine heart mitochondria. J Biol Chem. 1987 Feb 15;262(5):2005–2015. [PubMed] [Google Scholar]
- Côté J., Renaud J., Ruiz-Carrillo A. Recognition of (dG)n.(dC)n sequences by endonuclease G. Characterization of the calf thymus nuclease. J Biol Chem. 1989 Feb 25;264(6):3301–3310. [PubMed] [Google Scholar]
- Côté J., Ruiz-Carrillo A. Primers for mitochondrial DNA replication generated by endonuclease G. Science. 1993 Aug 6;261(5122):765–769. doi: 10.1126/science.7688144. [DOI] [PubMed] [Google Scholar]
- Engel P., Ullah A. H. Purification and characterization of an endonuclease (E.C. 3.1.30.1) from Streptomyces tendae. Prep Biochem. 1988;18(2):137–152. doi: 10.1080/00327488808062517. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Detection of triple-helix related structures adopted by poly(dG)-poly(dC) sequences in supercoiled plasmid DNA. Nucleic Acids Res. 1991 Aug 11;19(15):4267–4271. doi: 10.1093/nar/19.15.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman I. R. DNA ligase: structure, mechanism, and function. Science. 1974 Nov 29;186(4166):790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- Low R. L., Buzan J. M., Couper C. L. The preference of the mitochondrial endonuclease for a conserved sequence block in mitochondrial DNA is highly conserved during mammalian evolution. Nucleic Acids Res. 1988 Jul 25;16(14A):6427–6445. doi: 10.1093/nar/16.14.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low R. L., Cummings O. W., King T. C. The bovine mitochondrial endonuclease prefers a conserved sequence in the displacement loop region of mitochondrial DNA. J Biol Chem. 1987 Nov 25;262(33):16164–16170. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenna W. G., Maio J. J., Brown F. L. Purification and properties of a mammalian endonuclease showing site-specific cleavage of DNA. J Biol Chem. 1981 Jun 25;256(12):6435–6443. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction enzymes and their isoschizomers. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2331–2365. doi: 10.1093/nar/18.suppl.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Wu R. Terminal transferase-catalyzed addition of nucleotides to the 3' termini of DNA. Methods Enzymol. 1980;65(1):43–62. doi: 10.1016/s0076-6879(80)65009-5. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Renaud J. Endonuclease G: a (dG)n X (dC)n-specific DNase from higher eukaryotes. EMBO J. 1987 Feb;6(2):401–407. doi: 10.1002/j.1460-2075.1987.tb04769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. D., Hurwitz J. Enzymatic breakage of deoxyribonucleic acid. II. Purification and properties of endonuclease IV from T4 phage-infected Escherichia coli. J Biol Chem. 1969 Nov 25;244(22):6192–6198. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Ogawara H. Deoxyribonucleases in Streptomyces. J Antibiot (Tokyo) 1980 Oct;33(10):1206–1207. doi: 10.7164/antibiotics.33.1206. [DOI] [PubMed] [Google Scholar]
- de los Reyes-Gavilan C. G., Aparicio J. F., Barbes C., Hardisson C., Sanchez J. An exocytoplasmic endonuclease with restriction function in Streptomyces antibioticus. J Bacteriol. 1988 Mar;170(3):1339–1345. doi: 10.1128/jb.170.3.1339-1345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Reyes-Gavilán C., Cal S., Barbés C., Hardisson C., Sánchez J. Nutritional regulation of differentiation and synthesis of an exocytoplasmic deoxyriboendonuclease in Streptomyces antibioticus. J Gen Microbiol. 1991 Feb;137(2):299–305. doi: 10.1099/00221287-137-2-299. [DOI] [PubMed] [Google Scholar]