Abstract
Cyclooxygenase (Cox) is a key enzyme in the biosynthesis of prostaglandins and, as such, is the target of non-steroidal anti-inflammatory drugs (NSAIDs). Two isoforms exist, being expressed constitutively (Cox-1), or inducibly in response to inflammatory mediators (Cox-2). Currently available NSAIDs inhibit both isoforms somewhat equipotently but selective Cox-2 inhibition may eliminate unwanted side effects. We have characterized the kinetic mechanisms of the interactions of purified recombinant human cyclooxygenase-1 and -2 (hCox-1, hCox-2) with the selective Cox-2 inhibitor N-(2-cyclohexyloxy-4-nitrophenyl)methanesulphonamide (NS-398) and some classical non-selective NSAIDs. NS-398, flurbiprofen, meclofenamic acid and indomethacin are time-dependent, irreversible inhibitors of hCox-2. The inhibition is consistent with a two-step process, involving an initial rapid equilibrium binding of enzyme and inhibitor, characterized by Ki, followed by the slow formation of a tightly bound enzyme-inhibitor complex, characterized by a first-order rate constant kon. NS-398 is a time-independent inhibitor of hCox-1, consistent with the formation of a reversible enzyme-inhibitor complex. Flurbiprofen, meclofenamic acid and indomethacin are also time-dependent inhibitors of hCox-1 and hence show little selectivity for one isoform over the other. Flufenamic acid is time independent towards both isoforms and is also non-selective. The high degree of selectivity of NS-398 towards Cox-2 results therefore from the difference in the nature of the time-dependency of inhibition of the two isoforms.
Full text
PDF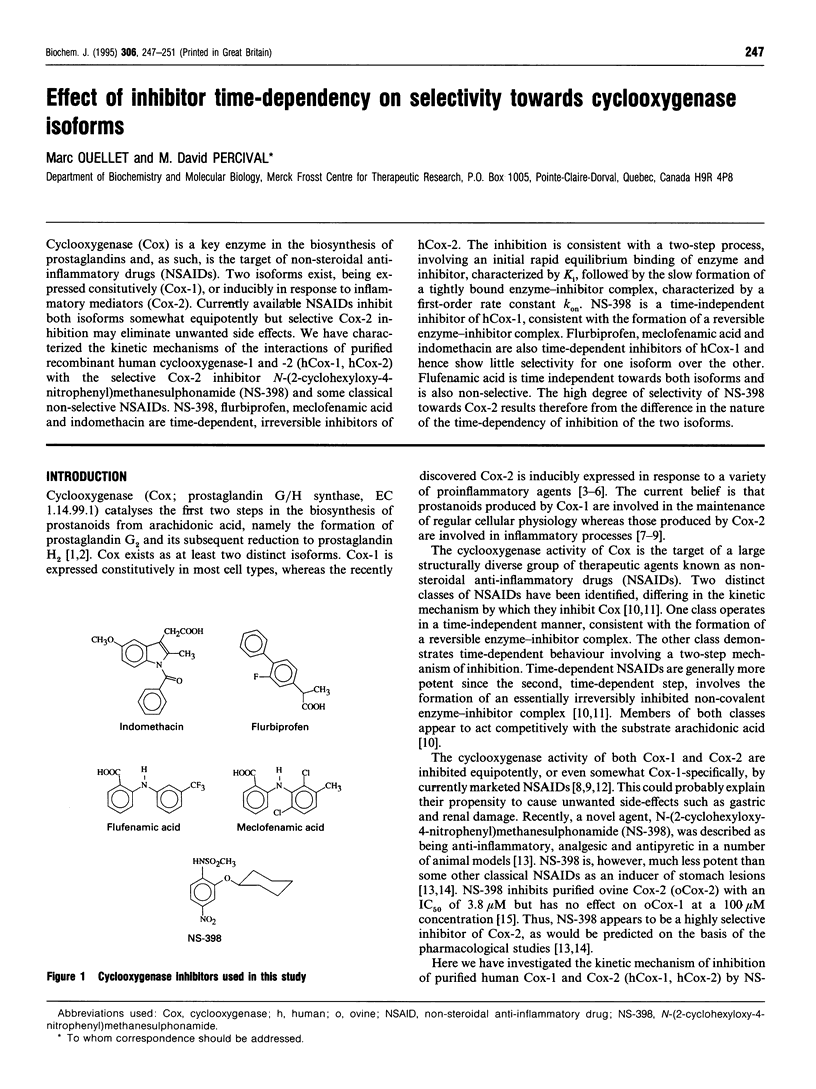


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., McDonald J. W. Reversible and irreversible inhibition of platelet cyclooxygenase and serotonin release by nonsteroidal antinflammatory drugs. Thromb Res. 1978 Dec;13(6):1057–1065. doi: 10.1016/0049-3848(78)90234-7. [DOI] [PubMed] [Google Scholar]
- Cromlish W. A., Payette P., Culp S. A., Ouellet M., Percival M. D., Kennedy B. P. High-level expression of active human cyclooxygenase-2 in insect cells. Arch Biochem Biophys. 1994 Oct;314(1):193–199. doi: 10.1006/abbi.1994.1429. [DOI] [PubMed] [Google Scholar]
- Futaki N., Takahashi S., Yokoyama M., Arai I., Higuchi S., Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994 Jan;47(1):55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Futaki N., Yoshikawa K., Hamasaka Y., Arai I., Higuchi S., Iizuka H., Otomo S. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen Pharmacol. 1993 Jan;24(1):105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Carlton D. P., McIntyre T. M., Zimmerman G. A., Prescott S. M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993 Apr 25;268(12):9049–9054. [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Kujubu D. A., Reddy S. T., Fletcher B. S., Herschman H. R. Expression of the protein product of the prostaglandin synthase-2/TIS10 gene in mitogen-stimulated Swiss 3T3 cells. J Biol Chem. 1993 Mar 15;268(8):5425–5430. [PubMed] [Google Scholar]
- Kulmacz R. J., Lands W. E. Stoichiometry and kinetics of the interaction of prostaglandin H synthase with anti-inflammatory agents. J Biol Chem. 1985 Oct 15;260(23):12572–12578. [PubMed] [Google Scholar]
- Kulmacz R. J., Pendleton R. B., Lands W. E. Interaction between peroxidase and cyclooxygenase activities in prostaglandin-endoperoxide synthase. Interpretation of reaction kinetics. J Biol Chem. 1994 Feb 25;269(8):5527–5536. [PubMed] [Google Scholar]
- Lee S. H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992 Dec 25;267(36):25934–25938. [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Manning P. T., Hauser S. D., Leahy K. M., Smith W. G., Isakson P. C., Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Mevkh A. T., Sud'ina G. F., Golub N. B., Varfolomeev S. D. Purification of prostaglandin H synthetase and a fluorometric assay for its activity. Anal Biochem. 1985 Oct;150(1):91–96. doi: 10.1016/0003-2697(85)90444-0. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Yamamoto S., Lands W. E. Effects of non-steroidal anti-inflammatory drugs on fatty acid cyclooxygenase and prostaglandin hydroperoxidase activities. Prostaglandins. 1982 May;23(5):743–757. [PubMed] [Google Scholar]
- O'Neill G. P., Mancini J. A., Kargman S., Yergey J., Kwan M. Y., Falgueyret J. P., Abramovitz M., Kennedy B. P., Ouellet M., Cromlish W. Overexpression of human prostaglandin G/H synthase-1 and -2 by recombinant vaccinia virus: inhibition by nonsteroidal anti-inflammatory drugs and biosynthesis of 15-hydroxyeicosatetraenoic acid. Mol Pharmacol. 1994 Feb;45(2):245–254. [PubMed] [Google Scholar]
- Percival M. D., Ouellet M., Vincent C. J., Yergey J. A., Kennedy B. P., O'Neill G. P. Purification and characterization of recombinant human cyclooxygenase-2. Arch Biochem Biophys. 1994 Nov 15;315(1):111–118. doi: 10.1006/abbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- Rane A., Oelz O., Frolich J. C., Seyberth H. W., Sweetman B. J., Watson J. T., Wilkinson G. R., Oates J. A. Relation between plasma concentration of indomethacin and its effect on prostaglandin synthesis and platelet aggregation in man. Clin Pharmacol Ther. 1978 Jun;23(6):658–668. doi: 10.1002/cpt1978236658. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Lands W. E. Structural requirements for time-dependent inhibition of prostaglandin biosynthesis by anti-inflammatory drugs. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4863–4865. doi: 10.1073/pnas.72.12.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Marnett L. J. Prostaglandin endoperoxide synthase: structure and catalysis. Biochim Biophys Acta. 1991 Apr 24;1083(1):1–17. doi: 10.1016/0005-2760(91)90119-3. [DOI] [PubMed] [Google Scholar]
- Walenga R. W., Wall S. F., Setty B. N., Stuart M. J. Time-dependent inhibition of platelet cyclooxygenase by indomethacin is slowly reversible. Prostaglandins. 1986 Apr;31(4):625–637. doi: 10.1016/0090-6980(86)90170-x. [DOI] [PubMed] [Google Scholar]


