Abstract
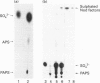
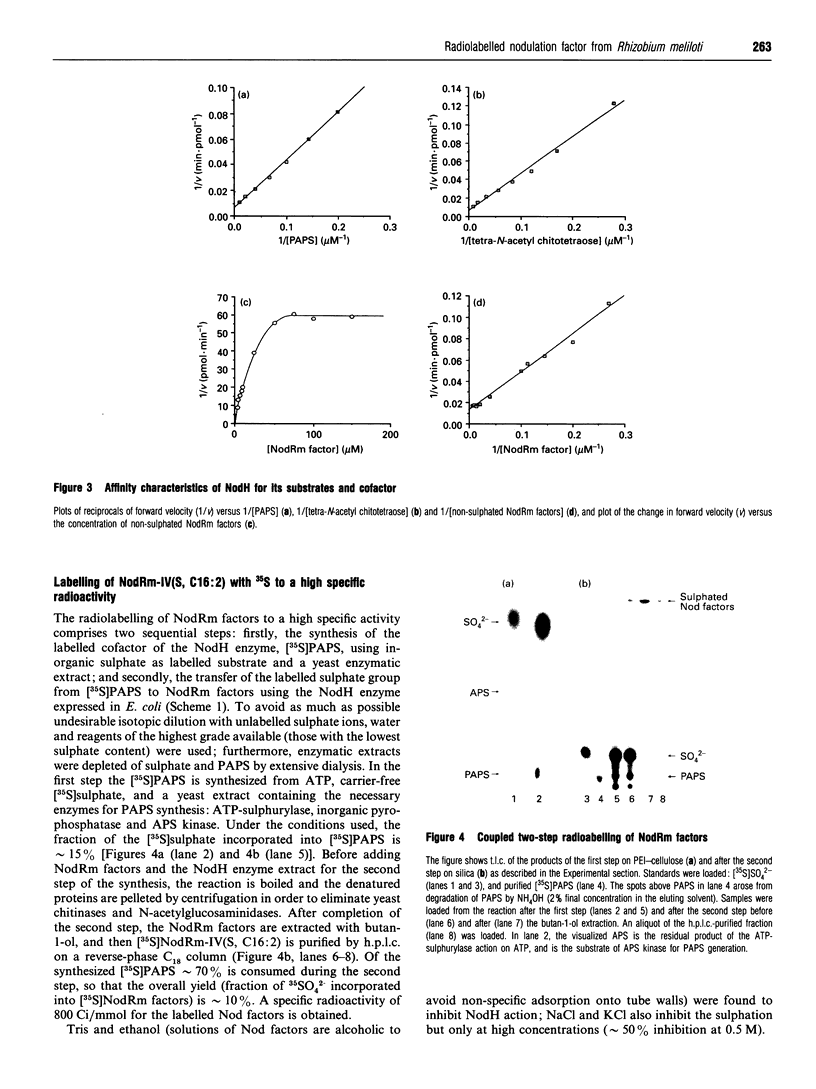
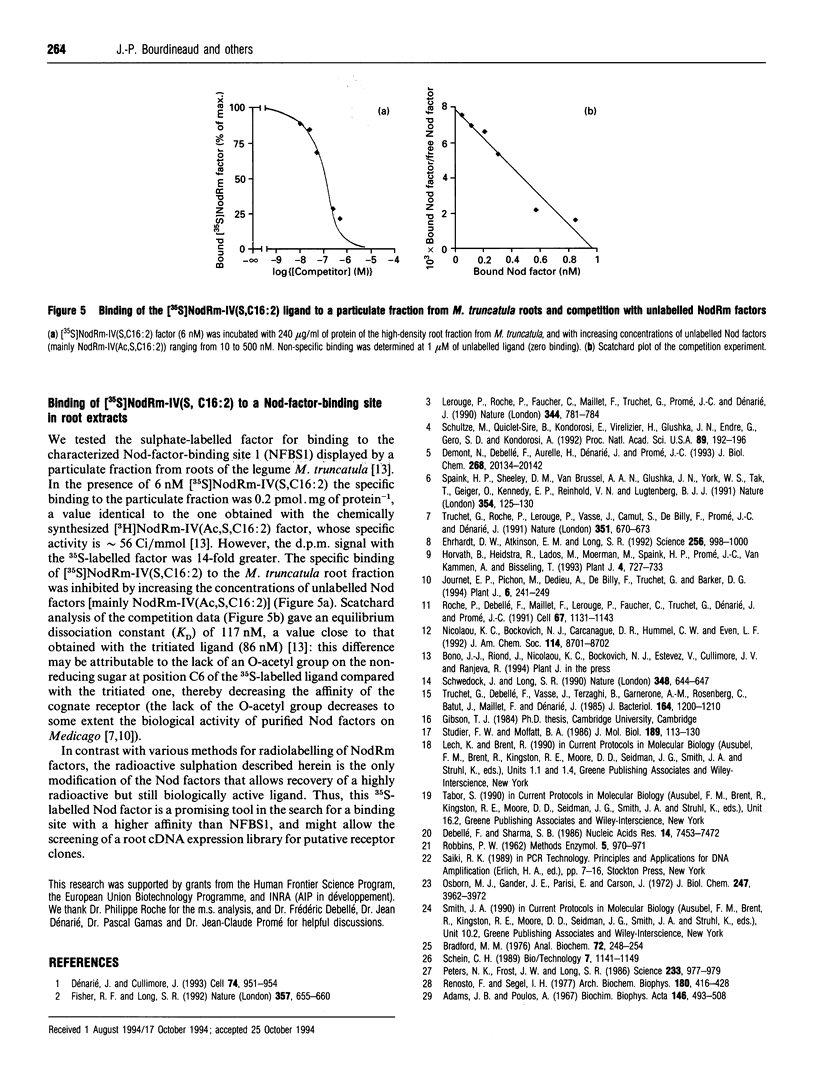
In this paper we describe the two-step coupled 35S-radiolabelling of the lipo-oligosaccharidic nodulation (Nod) factors of the bacterium Rhizobium meliloti to a specific radioactivity of 800 Ci/mmol. These radiolabelled Nod factors bind to a particulate fraction from roots of the bacterium's symbiotic host, Medicago truncatula, with an equilibrium dissociation constant (KD) of 117 nM, similar to that observed with a synthetic tritiated ligand. The first step of the 35S-labelling involves the synthesis of 3'-phosphoadenosine 5'-phospho[35S]sulphate ([35S]PAPS) from ATP and [35S]sulphate using yeast enzymes. The second step exploits the sulphotransferase activity of the R. meliloti NodH protein, which has been expressed in Escherichia coli, to transfer the labelled sulphate group from PAPS to non-sulphated Nod factors. This enzyme was found to be active in E. coli cultured at 18 degrees C but not 37 degrees C. NodH could also transfer the sulphate group from PAPS to a model substrate, tetra-N-acetyl chitotetraose, with apparent Km values of 56 and 70 microM respectively, and exhibited an apparent Km value for non-sulphated Nod factors of 28 microM. Coupling the two steps of the radiolabelling resulted in an efficiency of 35S incorporation from inorganic sulphate to the Nod factors of approximately 10%. These labelled factors will be a valuable tool in the search for high-affinity receptors for the lipo-oligosaccharidic nodulation factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. B., Poulos A. Enzymic synthesis of steroid sulphates. 3. Isolation and properties of estrogen sulphotransferase of bovine adrenal glands. Biochim Biophys Acta. 1967;146(2):493–508. doi: 10.1016/0005-2744(67)90233-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Debellé F., Sharma S. B. Nucleotide sequence of Rhizobium meliloti RCR2011 genes involved in host specificity of nodulation. Nucleic Acids Res. 1986 Sep 25;14(18):7453–7472. doi: 10.1093/nar/14.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demont N., Debellé F., Aurelle H., Dénarié J., Promé J. C. Role of the Rhizobium meliloti nodF and nodE genes in the biosynthesis of lipo-oligosaccharidic nodulation factors. J Biol Chem. 1993 Sep 25;268(27):20134–20142. [PubMed] [Google Scholar]
- Dénarié J., Cullimore J. Lipo-oligosaccharide nodulation factors: a minireview new class of signaling molecules mediating recognition and morphogenesis. Cell. 1993 Sep 24;74(6):951–954. doi: 10.1016/0092-8674(93)90717-5. [DOI] [PubMed] [Google Scholar]
- Ehrhardt D. W., Atkinson E. M., Long S. R. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992 May 15;256(5059):998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Long S. R. Rhizobium--plant signal exchange. Nature. 1992 Jun 25;357(6380):655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Horvath B., Heidstra R., Lados M., Moerman M., Spaink H. P., Promé J. C., van Kammen A., Bisseling T. Lipo-oligosaccharides of Rhizobium induce infection-related early nodulin gene expression in pea root hairs. Plant J. 1993 Oct;4(4):727–733. doi: 10.1046/j.1365-313x.1993.04040727.x. [DOI] [PubMed] [Google Scholar]
- Journet E. P., Pichon M., Dedieu A., de Billy F., Truchet G., Barker D. G. Rhizobium meliloti Nod factors elicit cell-specific transcription of the ENOD12 gene in transgenic alfalfa. Plant J. 1994 Aug;6(2):241–249. doi: 10.1046/j.1365-313x.1994.6020241.x. [DOI] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Renosto F., Segel I. H. Choline sulfokinase of Penicillium chrysogenum: partial purification and kinetic mechanism. Arch Biochem Biophys. 1977 Apr 30;180(2):416–428. doi: 10.1016/0003-9861(77)90056-x. [DOI] [PubMed] [Google Scholar]
- Roche P., Debellé F., Maillet F., Lerouge P., Faucher C., Truchet G., Dénarié J., Promé J. C. Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell. 1991 Dec 20;67(6):1131–1143. doi: 10.1016/0092-8674(91)90290-f. [DOI] [PubMed] [Google Scholar]
- Schultze M., Quiclet-Sire B., Kondorosi E., Virelizer H., Glushka J. N., Endre G., Géro S. D., Kondorosi A. Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):192–196. doi: 10.1073/pnas.89.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedock J., Long S. R. ATP sulphurylase activity of the nodP and nodQ gene products of Rhizobium meliloti. Nature. 1990 Dec 13;348(6302):644–647. doi: 10.1038/348644a0. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Truchet G., Debellé F., Vasse J., Terzaghi B., Garnerone A. M., Rosenberg C., Batut J., Maillet F., Dénarié J. Identification of a Rhizobium meliloti pSym2011 region controlling the host specificity of root hair curling and nodulation. J Bacteriol. 1985 Dec;164(3):1200–1210. doi: 10.1128/jb.164.3.1200-1210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]