Abstract
Progranulin (PGRN), a multifunctional growth factor-like protein expressed by a variety of cell types, serves an important function in the physiologic and pathologic processes of fibrotic diseases, including wound healing and the inflammatory response. PGRN was discovered to inhibit pro-inflammation effect by competing with tumor necrosis factor-alpha (TNF-α) binding to TNF receptors. Notably, excessive tissue repair in the development of inflammation causes tissue fibrosis. Previous investigations have indicated the significance of PGRN in regulating inflammatory responses. Recently, multiple studies have shown that PGRN was linked to fibrogenesis, and was considered to monitor the formation of fibrosis in multiple organs, including liver, cardiovascular, lung and skin. This paper is a comprehensive review summarizing our current knowledge of PGRN, from its discovery to the role in fibrosis. This is followed by an in-depth look at the characteristics of PGRN, consisting of its structure, basic function and intracellular signaling. Finally, we will discuss the potential of PGRN in the diagnosis and treatment of fibrosis.
Key words: Progranulin, Fibrotic disease, Fibrosis, Inflammation, Biomarker, Therapeutic target, PGRN/TNFR interaction, Signaling pathway
Graphical abstract
Progranulin (PGRN) is a promising biomarker and therapeutic target for fibrotic diseases. PGRN can act both as a pro-fibrotic and as anti-fibrotic factor in several fibrotic diseases.
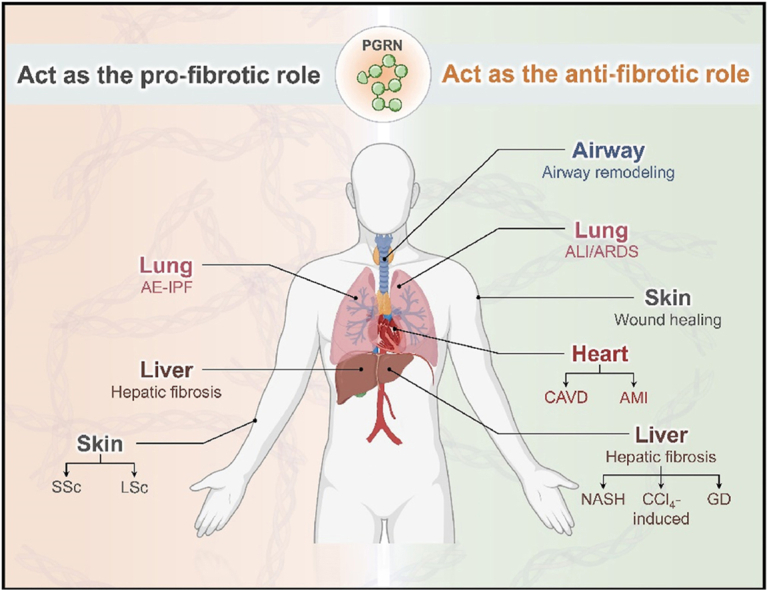
1. Introduction
Fibrosis is a consequence of the tissue remodeling response characterized by the deposition of collagen and other extracellular matrix (ECM) molecules1,2. If highly progressive and not effectively controlled, fibrosis ultimately results in organ malfunction and death. The most common clinical fibrotic diseases are systemic diseases; systemic sclerosis (SSc); chemical and radiation-induced fibrotic diseases; and organ-specific pathologies such as cardiac, liver, pulmonary, skin and renal fibrosis3. According to relevant data, cirrhosis is a leading cause of death worldwide—it was associated with 2.4% of global deaths in 20194. Moreover, idiopathic pulmonary fibrosis (IPF) accounts for over 16,000 deaths per year in the United States5. In America, almost 45% of deaths from various disorders can be attributed to tissue fibroproliferative diseases6. In addition, the total annual global incidence of fibrotic diseases is nearly 4.968% of the population per year7. The incidence of fibrotic disease and the number of fibrosis-related deaths continue to increase with an ever-expanding aging population8. Although the etiologies of different fibrotic diseases vary, the pathogenesis of these diseases is consistent. The main pathogenesis is an inordinate accumulation of fibrous connective tissue around inflamed or damaged tissue, leading to an excessive increase in fibronectin and collagen in the ECM and the differentiation of fibroblasts into myofibroblasts, causing permanent scarring and organ dysfunction9,10. Notably, fibroblast activation is regulated by various secreted soluble factors. It has been reported that Progranulin (PGRN) acts as a pleiotropic growth factor and its reduction promotes fibrogenesis in skin wound healing11. Hence, further elucidation of the mechanism of fibrotic disorders and identification of more effective therapeutic targets for extra fibrosis are needed.
PGRN, a pleiotropic growth factor-like protein produced in assorted tissues, is involved in diverse pathological and physiologic processes, including embryogenesis, wound healing, host defense, tumorigenesis, and cartilage degeneration12,13. Furthermore, PGRN has a well-accepted role in inflammatory cell proliferation and has been demonstrated to be involved in the process of immune diseases14. It has been reported that overexpressed serum PGRN acts as an independent predictor of the extent of liver fibrosis in nonalcoholic fatty liver disease (NAFLD) patients15. Similarly, in models of carbon tetrachloride-induced liver fibrosis and methionine-choline-deficient diet-induced nonalcoholic steatohepatitis, PGRN exhibited protective effects against liver damage, fibrosis and inflammation through inhibiting the phosphorylation of nuclear transcription factor kappa B (NF-κB)16. These results increase the understanding of the importance of PGRN in fibrotic diseases and strongly indicate that PGRN is a novel biomarker and treatment target in fibrotic diseases.
The tissue remodeling response is highly dynamic and the cells involved in this process are continuously renewed throughout their lifetime. These findings are consistent with the findings of previous reports on external factors or internal signaling pathways, and accumulating research has explored the role of PGRN in tissue remodeling through the targeting of different molecules17,18. Therefore, we reviewed recent studies linking PGRN to fibrotic diseases. We first summarize the knowledge of PGRN, and subsequently provide insight into the functions and regulatory roles of PGRN in fibrotic diseases. Finally, the clinical relevance of PGRN regulation in fibrotic disorders was also explored to provide insight into the development of therapeutic strategies.
2. Overview of PGRN
2.1. Basic structure of PGRN
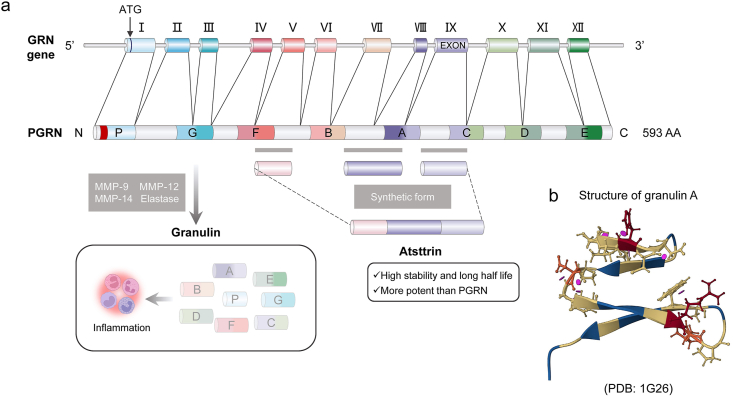
PGRN, also referred to as granulin-epithelin precursor19, acrogranin20, proepithelin, GP88, and PC-cell derived growth factor21, is an autocrine multifunctional growth factor that includes 593 amino acids and has a molecular weight of nearly 75–80 kDa that contributes to modulating cell proliferation and wound repair22. It is encoded by the GRN gene and is located at chromosomal region 17q21.32; this gene 12 exons and results in three isoforms14. PGRN consists of 7½ domains of a cysteine-rich motif (CX5-6CX5CCX8CCX6CCXDX2HCCPX4CX5-6C, X: any amino acid) in the order P-G-F-B-A-C-D-E, where P is the incomplete motif and A-G are full repeats23, which are connected by six disulfide bonds, forming four sequentially trapezoidal folded β hairpins structures in the spatial structure24 (Fig. 1). In addition, PGRN is decidedly expressed in certain types of neurons25, and macrophages26 and is also expressed in a broad range of other cells and tissues, containing chondrocytes27, adipose tissue28, hematopoietic cells29, and immune cells30, including dendritic cells and T cells. In 1998, for the first time, PRGN was demonstrated to promote cell proliferation by directly binding to fibroblasts31.
Figure 1.
The structure and production of PGRN. (a) PGRN gene (GRN), located on chromosome 17q21.32, consists of 12 exons. PGRN is formed by 7.5 domains (a granulin motif in the order of P–G–F–B–A–C–D–E, where A–G are full repeats and P is the half-motif) of a 12-cysteine motif, which cleavages producing GRNs that promotes inflammation. Atsttrin is the synthetic form derived from FAC domain of PGRN (from Ref. 129). (b) Human granulin A structure (from protein data bank, https://www.rcsb.org/, PDB ID, 1G26). PGRN, progranulin; AA, amino acid; MMP, matrix metalloproteinases.
2.2. Biological functions of PGRN
PGRN is understood to play an essential role in various physiological and disease processes, including early embryonic development, inflammation and wound healing32. The multiple roles of PGRN are mediated by various related proteins33. Uniquely, PGRN can bind to a wide range of proteins at distinct levels, ranging from the extracellular fluid and the ECM to intracellular components, including the nucleus and cytoplasm34. This adaptability is likely a result of the exceptional structure of the PGRN. For instance, the PGRN F-B domain can be identified by Laminin G and the EGF-like domain of Perlecan domain V35. Interestingly, Laminin and Perlecan are the main components of the ECM. So far, more than 20 proteins have been identified as binding partners of PGRN36, 37, 38, 39, 40, 41; among them are several cell surface proteins, including tumor necrosis factor receptors (TNFRs)42, sortilin 143 and Toll-like receptor 944. According to a previous study, PGRN was found to be a novel ligand that blocks TNF-alpha (TNF-α)-mediated signaling pathways by competing with TNF-α for binding to TNFR1/2, hence inhibiting its proinflammatory effects45. Moreover, secretory leukocyte protease inhibitor binds directly to PGRN, blocking the proteolysis by elastase46. Increasing evidence has indicated that PGRN is digested into 6 kDa GRN peptides by many proteases, consisting of matrix metalloproteinases 9 and 12, elastase and protease 336,47, 48, 49. However, GRN, as opposed to PGRN, promotes the expression of neutrophil-attracting chemokines during inflammation50. Therefore, maintaining a balance between PGRNs and GRNs is also an important mechanism through which PGRNs characterize their biological functions.
In many types of diseases, inflammation is the trigger for fibrosis51. Activated inflammation leads to the overexpression of inflammatory mediators52,53. Fibroblasts and other mesenchymal cells are subsequently transformed to myofibroblasts via the enrichment of fibrotic cytokines, where they secrete ECM components54. In the normal wound healing response, activated myofibroblasts are removed from the wound site by apoptosis following injury repair55. Conversely, during the fibrotic process, myofibroblasts fail to undergo apoptosis and are subsequently activated, ultimately contributing to disproportionate ECM deposition56. While PGRN is one of the main anti-inflammatory factors, the specific function of PGRN may differ according to the stage and components involved in inflammatory responses33. For example, PGRN affects the development of macrophages, neutrophils, blood vessels, and fibroblasts in acute skin damage57, but in acute infection, PGRN represses lipopolysaccharide (LPS)-mediated interleukin (IL)-6, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) cytokine release from macrophages58. Furthermore, the results of several studies revealed that TNF-α has profibrotic effects in various animal models generated from TNF-α blockers or TNF-receptor deficient mice59, 60, 61. Therefore, PGRN competitively binds to TNF-α receptors, which may be an underlying mechanism for the inhibition of fibrosis.
As mentioned above, PGRN has potent inflammatory effects in multiple animal models, and thus can downregulate the expression of proinflammatory molecules, leading to decreased injury correlated with fibrosis16,62. Moreover, although the exact mechanisms underlying the role of PGRN in some fibrotic diseases have largely not been elucidated, increasing evidence has demonstrated that PGRN is involved in fibrosis11,16,17. In brief, PGRN is involved in the generation of fibrosis-related cytokines and products, and plays a significant role in human fibrotic diseases.
3. PGRN in fibrotic diseases
Fibrotic diseases are characterized by the activation of ECM-producing myofibroblasts, and include SSc, liver fibrosis, kidney fibrosis and other related diseases63. Although increasing in-depth research has focused on these diseases, the underlying mechanisms have not been fully elucidated, hampering advances in biomarker and therapeutic target research for fibrotic disease. As a consequence, there is an urgent need to obtain a better understanding of the mechanisms underlying fibrosis to design new therapies that postpone or prevent disease progression. Additionally, current works have revealed that PGRN participates in the pathogenesis and development of them, including liver fibrosis16 and cardiovascular fibrosis64,65, etc.17 (Table 1). Recent studies have shown that the expression level of PGRN was increased in several fibrotic diseases and exhibited anti-inflammatory effects, thereby inhibiting organ fibrosis.
Table 1.
Progranulin in fibrotic diseases.
| Organ | Disease | Subject | Expression level | Link to PGRN | PMID |
|---|---|---|---|---|---|
| Liver | NAFLD | NAFLD patients | ↑ | An independent predictor of the degree of hepatic fibrosis in patients | 22045426 |
| MCD-induced NASH | Mice RAW264.7, HepG2, Huh7 |
↑ | PGRN attenuated liver fibrosis by reducing hepatic steatosis and injury | 31591383 | |
| CCl4-induced liver fibrosis | Mice RAW264.7, HepG2, Huh7, human primary stellate cells |
↑ | PGRN reduced liver fibrosis by inhibiting inflammation | 31591383 | |
| GD | GD patients | ↓ | Negatively correlated with clinical disease severity and liver stiffness | 32652633 | |
| Thioacetamide-induced liver cirrhosis | Rats | ↑ | PGRN was upregulated in cirrhotic livers in vivo | 19784507 | |
| Heart | CAVD | Patients | ↑ | PGRN significantly reduced the fibrosis markers | 32247641 |
| hVICs, pVICs | ↓ | ||||
| grn−/−MEFs | NA | ||||
| AMI | Mice, rabbits | ↑ | PGRN decreased fibrosis size in myocardium | 32678228 | |
| Lung | AE-IPF | AE-IPF patients | ↑ | A possible marker of disease activity in AE-IPF | 34462814 |
| ALI | Mice | ↑↓a | Pro-inflammatory mediators were decreased | 33941562 | |
| Mice RAW164.7 |
NA | Anti-inflammatory factor was upregulated | 32565732 | ||
| Mice | ↑↓ | PGRN/TNFR2 interaction played a protective role in ALI | 22969170 | ||
| Bronchus | HDM-induced chronic asthma | Mice BEAS-2B |
NA | Administration mitigated inflammation and fibrosis | 34408470 |
| Skin | Skin wound healing | Mice | ↑ | Downregulation enhanced the fibrosis degree | 30561754 |
| SSc | SSc patients Mice |
↑ | Increased in diseased tissue, inhibition improved disease in mice models | 31108104 | |
| LSc | LSc patients Human dermal fibroblasts |
↑ | Overproduction induced the pro-fibrotic phenotype in dermal fibroblasts | 30268392 |
“NA” indilegecates that changes in the expression level are not mentioned in the reference.
NAFLD, nonalcoholic fatty liver disease; MCD, methionine-choline-deficient diet; NASH, non-alcoholic steatohepatitis; CCl4, carbon tetrachloride; GD, Gaucher disease; CAVD, calcific aortic valve disease; AMI, acute myocardial infarction; AE-IPF, acute exacerbation of idiopathic pulmonary fibrosis; ALI, acute lung injury; HDM, house dust mite; SSc, systemic sclerosis; LSc, localized scleroderma; PGRN, progranulin; TNFR2, tumor necrosis factor-alpha receptor 2.
“↑↓” indicates that the expression level of PGRN increases first and then decreases significantly.
3.1. Hepatic fibrosis
Hepatic fibrosis is a reversible wound-healing response characterized by the abnormal accumulation of ECM proteins containing collagen owing to most chronic liver injuries66,67. In terms of the complex and diverse pathogenesis of liver fibrosis, although the exact function of PGRN in this disease is unknown, there is no doubt that PGRN has a great influence on the progression and pathogenesis of liver fibrosis16,68 (Fig. 2).
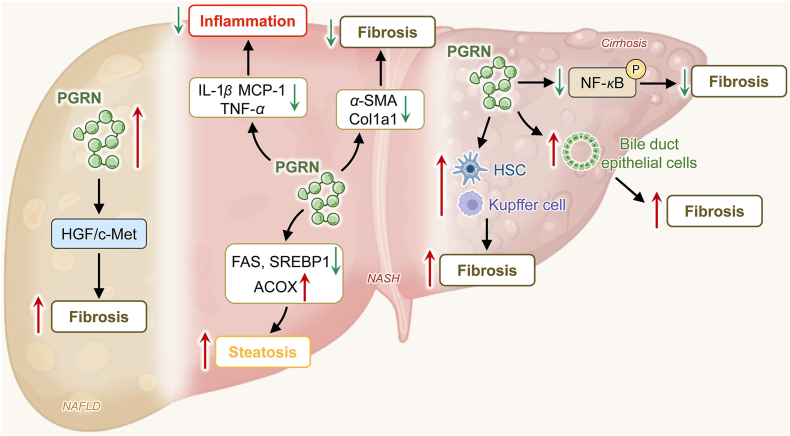
Figure 2.
PGRN in hepatic fibrosis. Upregulated serum PGRN acted as an independent marker of liver fibrosis in patients with NAFLD, which could occur via the regulation of the HGF/c-Met signaling pathway. Moreover, PGRN attenuated liver fibrosis by downregulating hepatic steatosis and inflammation in NASH. PGRN was up-regulated in cirrhotic livers and positivity was strong in hepatic areas of bile duct proliferation and in fibrous septa, suggesting the involvement of this growth factor in bile duct epithelial cell and non-parenchymal cell proliferation, which contributes to the fibrotic process. PGRN, progranulin; NAFLD, nonalcoholic fatty liver disease; HGF, hepatocyte growth factor; IL-1β, interleukin-1 beta; MCP-1, monocyte chemoattractant protein-1; α-SMA, α-smooth muscle actin; FAS, fatty acid synthase; SREBP1, sterol regulatory element-binding protein-1; ACOX, (acyl-CoA oxidase; HSC, hepatic stellate cell; NF-κB, nuclear transcription factor kappa B.
In a study consisting of 95 patients with biopsy-proven NAFLD and 80 age- and sex-matched controls, Yilmaz et al. found that the serum PGRN concentration was positively related to the degree of hepatocyte fibrosis. Moreover, Yilmaz et al.15 illustrated that PGRN could serve as a predictor of hepatocyte fibrosis in NAFLD patients after adjustment for a broad spectrum of probable confounders. Nevertheless, this study did not elucidate the causal relationship between serum PGRN levels and the occurrence of liver fibrosis. Notably, several studies have shown that PGRN suppresses hepatic fibrosis16,69,70. Yoo et al.16 have suggested that PGRN could attenuate liver fibrosis by reducing the inflammatory response. Researchers have investigated the role of PGRN by establishing two models of chronic liver disorders, methionine-choline-deficient diet-induced nonalcoholic steatohepatitis and carbon tetrachloride-induced liver fibrosis. Several studies have shown that disturbances in macrophage function can result in aberrant repair, which can lead to the progressionof pathological fibrosis71. In both models, PGRN decreased macrophage activation and infiltration, contributing to reduced hepatic fibrosis. Moreover, the results of the in vitro and in vivo experiments indicated that the expression levels of TNF-α, interleukin-1beta (IL-1β), MCP-1, alpha-smooth muscle actin (α-SMA), and collagen type I alpha 1 chain were significantly downregulated in the PGRN-treated group than in the blank group. Additionally, in Gaucher disease (GD) patients, Tantawy et al.69 reported a further negative correlation between serum PGRN and liver stiffness as well as the potential of PGRN as a biomarker of liver fibrosis in GD patients.
Conversely, a previous study by Guerra et al.72 revealed that both PGRN protein and mRNA positivity were unmistakable in hepatic areas of bile duct proliferation and in fibrous septa by using in situ hybridization and immunohistochemistry. These results indicated that growth factors contributed to the fibrotic process. Several reasons may explain the conflicting results: First, different modeling methods could cause deviations in the results. Second, animal experiments cannot completely explain the conditions in humans. Furthermore, sample size and group limitations may partially account for such contradictory findings.
3.2. Cardiovascular fibrosis
3.2.1. Calcific aortic valve disease
Calcific aortic valve disease is a universal valvular heart diseases that encompasses a disorder spectrum ranging from aortic valve sclerosis to gross left ventricular (LV) outflow obstruction by calcific aortic valve stenosis, which is characterized by the presence of mineralized nodules and fibrosis (i.e., progressive mineralization and fibrosis in the aortic leaflets)73,74. Many studies on PGRN expression have shown that PGRN is produced and secreted from macrophages, endothelial cells, neuronal cells, etc.75. Of note, one study by Huang et al.64 provided the first report to state that PGRN is expressed in valve interstitial cells. In addition, data from the study showed that PGRN expression increased in valves from calcific aortic valve disease patients but decreased in primary porcine valve interstitial cells. The results suggested that increasing concentrations of PGRN elicited continuous reductions in intracellular TNF-α expression until the maximum peak was reached in response to 800 ng/mL PGRN, and the fibrosis marker α-SMA was also downregulated. As mentioned above, the expression of PGRN has also been reported to be upregulated under some conditions, including hypoxia76 and traumatic brain injury77. Therefore, the overexpression of PGRN may be the consequence of a compensatory response, as analyzed by Yin et al.78 Taken together, these findings suggest that PGRN might constitute a novel biomarker for mitigating valve fibrosis.
3.2.2. Acute myocardial infarction
Acute myocardial infarction (AMI) can present as myocardial necrosis caused by acute thrombotic obstructions of coronary artery blood flow79. AMI arises when blood ceases to suddenly flow to a part of the heart, and the myocardium is injured owing to a lack of oxygen supply80. AMI always results in varying degrees of ventricular remodeling. Approximately three days post-MI, the beginning of fibroblast proliferation and replacement fibrosis cause the formation of a collagen I-rich scar81. Unlike healthy myocardium, the fibrotic scar is electrically inert and less contractile, exacerbating myocardial fibrosis and deteriorating cardiac function, which leads to no fundamental improvement in clinical symptoms or prognosis82. As such, the prevention of myocardial fibrosis post-AMI is essential for patient survival. It has been demonstrated that PGRN ameliorates fibrosis after myocardial ischemia/reperfusion (I/R) in rabbits65. To our knowledge, the LV internal diameter is correlated with an index of adverse cardiac repair83. Sasaki et al.65 have suggested that recombinant PGRN markedly weakens the deterioration of the internal diameter of the LV at diastole and systole according to echocardiography analysis. Moreover, Masson's trichrome staining revealed that, compared with vehicle, PGRN strongly reduced the extent of fibrosis in the myocardium by 10% after myocardial I/R. In conclusion, PGRN might have therapeutic potential for fibrosis in myocardial I/R injury.
3.3. Respiratory system fibrosis
3.3.1. Idiopathic pulmonary fibrosis
IPF is a progressive chronic interstitial pneumonia of unknown pathogenesis characterized by incessant scarring of the lung parenchyma triggering reduced quality of life and earlier mortality84. In particular, acute exacerbation of IPF (AE-IPF) is an acute, clinically significant, respiratory exacerbation of unexplained causes85 and may even occur in individuals with limited fibrosis and well-preserved lung function86. Interestingly, a study based on patients with AE-IPF hospitalized at Hainan General Hospital between January 2017 and June 2020, demonstrated that PGRN expression in the study group was dramatically greater than that in matched controls12. According to guidelines from the European Respiratory and American Thoracic Society, decreased diffusing capacity of carbon monoxide (DLCO) is often observed in patients with structural lung diseases, consisting of chronic obstructive lung disease and interstitial lung disease (ILD)87. Hence, DLCO is used to indicated to measure the progression of pulmonary fibrosis. Xie et al.12 has also found an obvious negative correlation between PGRN and DLCO, suggesting that the PGRN concentration increases as fibrosis worsens. As discussed previously, the mechanism underlying the increase in PGRN may involve in the inflammatory response to AE-IPF, and PGRN could be useful as a clinical marker of AE-IPF (Fig. 3). Another study has shown that serum PGRN levels were significantly higher in non-IPF ILD patients compared to healthy subjects and stable IPF patients88. The difference can be explained by the mechanism of PGRN in acute exacerbation.
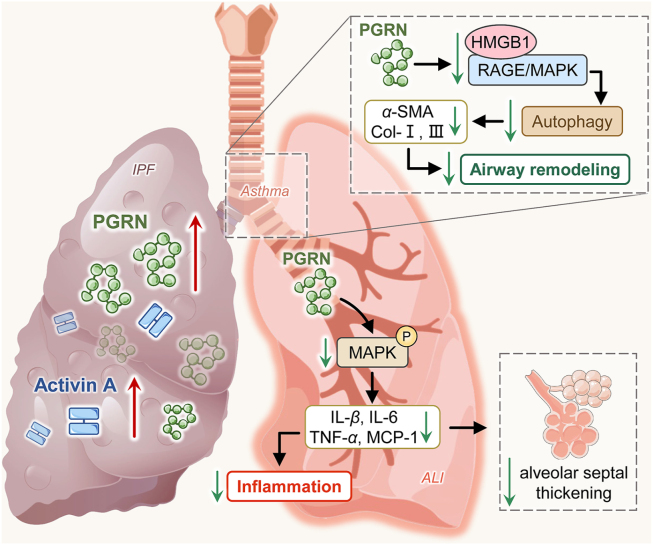
Figure 3.
PGRN in respiratory system fibrosis. In AE-IPF, PGRN concentration increased as fibrosis worsen, which functioned as possible markers of disease activity together with activin A. For patients with asthma, PGRN significantly reduced collagen-I, α-SMA and collagen-III in TGF-β-induced airway epithelial cells via autophagy, thereby preventing airway remodeling and improving asthma. Furthermore, PGRN attenuated the formation of inflammatory molecules and alveolar septal thickening by inhibiting the MAPK signaling pathway in ALI. PGRN, progranulin; HMGB1, high-mobility group box protein 1; RAGE, The receptor for advanced glycation end product; MAPK, mitogen-activated protein kinase; α-SMA, α-smooth muscle actin; IL-1β, interleukin-1 beta; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-alpha.
3.3.2. Asthma
Asthma is a chronic, heterogeneous condition that may result from exposure to allergens or other environmental irritants89,90. It has been demonstrated that airway remodeling is a cardinal feature of asthma in which airways undergo structural changes, and are capable of causing variable airflow limitation in patients with asthma91. Additionally, previous studies demonstrated that fibrosis is an important characteristic of airway remodeling92,93. Earlier work by Minshall et al. confirmed that a progressive increase in fibrosis was correlated with increasing severity of asthma94. Thus, the suppression of airway fibrosis would be beneficial for relieving airway remodeling, thereby improving asthma symptoms and prognosis. A study conducted by Liu et al.17 indicated that PGRN protected against remodel of the asthmatic airway by inhibiting autophagy. The findings indicated that the increased mRNA expression of the fibrosis-related genes collagen-I, α-SMA, transforming growth factor beta 1 (TGF-β1) and matrix metalloproteinase-9 (MMP9) in house dust mite-induced mice was greatly suppressed by PGRN treatment. Meanwhile, PGRN dramatically reduced the expression levels of collagen-I, α-SMA and collagen-III induced by TGF-β1 in BEAS-2B cells (a cell from the human airway epithelium)17. Overall, PGRN may function as a potential treatment option for chronic asthma (Fig. 3).
3.3.3. Acute lung injury/acute respiratory distress syndrome
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is characterized by diffuse alveolar injury, which causes excessive pulmonary inflammation and pathological changes in lung tissues95, 96, 97. Previous observations suggested that injury and repair mechanisms occur synchronously rather than in sequentially98,99. More importantly, Marshall et al. demonstrated that 24 h following disease onset, the level of N-terminal procollagen peptide III, an indicator of collagen turnover, was significantly greater in the bronchoalveolar lavage fluid (BALF) of ARDS patients than in that of controls100. Accumulated evidence has shown that pulmonary tissue morphology, including fibrin deposition and alveolar wall thickening, is altered from a normal state to fibrosis in ALI model mice101,102. Therefore, fibrosis may be an early response to ALI/ARDS and a crucial therapeutic target for improving patient prognosis. It is generally accepted that PGRN has antifibrotic effects65,103. Nevertheless, the mechanisms of PGRN in ALI/ARDS remained uncertain. In a study by Lu et al.102, PGRN markedly suppressed the formation of inflammatory molecules including IL-1β, IL-6, TNF-α, and MCP-1. In accordance with the theory that dysregulated or chronic inflammation may contribute to the progression of pathological fibrosis or scarring104, the above study revealed that treatment with PGRN also significantly attenuated alveolar septal thickening and transparent membrane formation. Similarly, Chen105 and Guo et al.106 also showed that PGRN could significantly reduce intra-alveolar fibrin in ALI mouse models. Apparently, the antifibrotic efficacy of PGRN in the ALI model indicates that PGRN is a promising therapeutic approach for fibrosis in ALI/ARDS (Fig. 3).
3.4. Skin fibrosis
As the body's main external barrier, the skin is a common site of tissue damage107. Following injury, a complex wound repair reaction occurs in the skin, which is generally imperfect, with some degree of fibrosis or scarring108. This outcome leads to the replacement of native tissue with dense connective tissue, ultimately resulting in loss of normal tissue function109. Although the effect of PGRN on the fibrosis process in skin remains poorly understood, the PGRN gene is expressed in dermal fibroblasts, endothelial cells and infiltrating leukocytes and is a mediator of the wound response110. Notably, data from a study by Li et al.11 found that the protein and mRNA levels of PGRN were elevated after injury and that the downregulation of PGRN augmented the fibrosis area, skin thickness and collagen I expression during cutaneous wound healing. Similarly, Li et al.18 demonstrated that PGRN upregulation decreased both the area and thickness of granulation/fibrosis tissue in a mouse model.
Nevertheless, this is inconsistent with the findings based on a study about on skin sclerosis carried out by Yang et al111. It is well known that SSc is a rare, complex and chronic connective tissue disease of unknown etiology, the cardinal symptom of which is skin fibrosis112,113. Using a bleomycin (BLM)-induced dermal fibrosis mouse model, researchers have shown that a decrease in PGRN expression ameliorates fibroblast activation and skin fibrosis. Additionally, compared with those in BLM-induced wild-type (WT) mice, there was a distinct reduction in dermal thickness and a great attenuation of collagen fibers in skin tissue in BLM-induced PGRN−/− mice 111. By coincidence, based on the current data about the double-edged role of TNF-α on fibrosis114,115, Miyagawa et al.116 investigated the impact of PGRN on localized scleroderma (LSc), the results of which indicated that PGRN overproduction aggravated skin fibrosis via blockade of the antifibrotic function of TNF-α on skin fibroblasts; the same was true for SSc. Interestingly, it was reported that PGRN can both act as an anti-inflammatory factor and a proinflammatory factor117. Thus, it was speculated that PGRN maintained the pro-fibrotic phenotype due to the special anti-fibrosis mechanism of SSc and LSc dermal fibroblasts.
Given that the above studies involved different disease models and complex pathogenesis, PGRN seems to have dual modes of action, such as acting as a promoter and inhibitor in the development of skin fibrosis. Overall, the present findings provide new insights into the complicated effects of PGRN in skin fibrotic disorders.
4. PGRN expression and mechanisms in fibrotic diseases
Previous studies have demonstrated that patients with fibrotic diseases exhibit elevated plasma PGRN levels compared with healthy individuals. A study carried out by Yilmaz et al. suggested that serum PGRN levels were significantly greater in NAFLD patients than in controls15. Moreover, in a recent cross-sectional study, serum PGRN levels were significantly greater in non-IPF ILD patients compared to healthy subjects and IPF. By coincidence, Xie et al.12 reported that PGRN levels were greater in AE-IPF patients than in healthy individuals, which indicates a possible pathogenetic role of PGRN in this disease, as noted by Tanaka et al.118. Therefore, it can be assumed that PGRN expression is lower in chronic and stable IPF patients compared to non-IPF ILD and AE-IPF patients, but still higher than that in the control population.
Analysis of the human promoter of the GRN revealed that its expression is likely regulated by inflammation119. Based on the data thus far, inflammation may be an important mechanism for bridging PGRN and fibrosis. There are potential factors such as IL-6120,121 and TNF-α121 that can influence serum PGRN levels. Although PGRN has been extensively studied as an anti-inflammatory factor, increased PGRN levels are likely to be a compensatory response to the inflammatory response77. Additionally, some researchers have speculated that altered levels of PGRN with hepatic fibrogenesis can occur via regulation of the hepatocyte growth factor/mesenchymal–epithelial transition factor system signaling pathway15.
5. Regulatory mechanism of PGRN in fibrotic diseases
5.1. PGRN signaling pathways
The global understanding of signal transduction regulatory networks in biological processes is highly important for exploring the molecular mechanisms of disease122, 123, 124. Notably, the onset and progression of fibrotic diseases are regulated by complex molecular mechanisms and different signaling pathways2, while our present understanding of fibrosis is incomplete, which causes the treatment of fibrosis is currently at a bottleneck stage. Hence, an increasing number of studies have focused on the effect of PGRN on fibrosis at the molecular level and revealed that mitogen-activated protein kinase (MAPK), the TGF-β/Smad pathway, and the NF-κB signaling pathway are activated during the development of fibrotic diseases (Fig. 4).
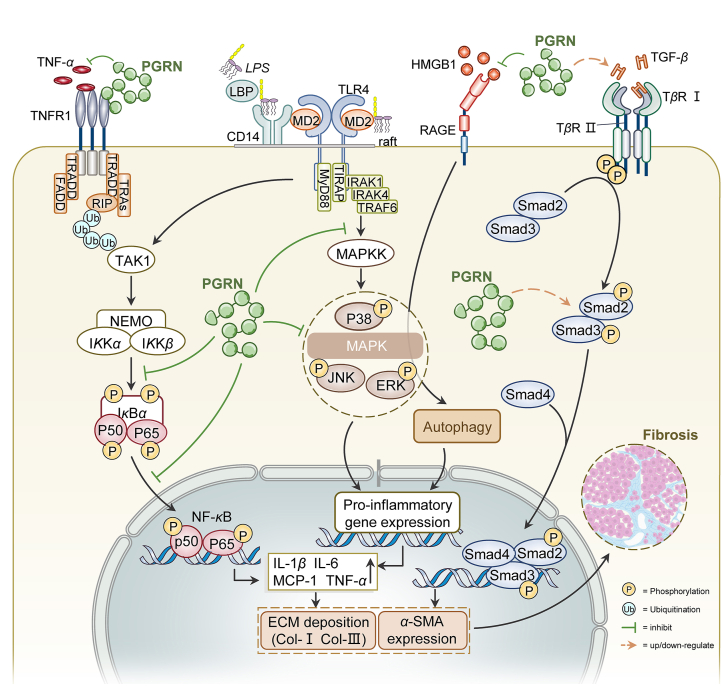
Figure 4.
Functions and signaling pathways associated with PGRN in fibrogenesis. (1) PGRN inhibited competitively TNF/TNFR1, followed by the suppression of the NF-κB pathway. Besides, PGRN significantly reduced phosphorylation of IкBα, p65 and the nucleus translocation of NF-κB p65. (2) PGRN also delayed the inflammatory process through inhibiting the increase of HMGB1 and the activation of RAGE/MAPKs signaling pathway. In another study about ALI, PGRN inhibited the activation of the MAPK signaling pathway via decreasing phosphorylation of ERK, JNK and p38. (3) PGRN played dual roles in modulating the TGF-β/Smad signaling pathway. Abbreviations: PGRN, progranulin; TNF-α, tumor necrosis factor-alpha; TNFR1, TNF receptor 1; TRADD, TNFR1-associated death domain protein; FADD, Fas-associated protein with death domain; TRAFs, TNFR-associated factors; RIP, receptor interacting protein; NF-κB, nuclear transcription factor kappa B; NEMO, NF-κB essential modulator; IL-1β, interleukin-1 beta; MCP-1, monocyte chemoattractant protein-1; α-SMA, againstα-smooth muscle actin; ECM, extracellular matrix; LPS, lipopolysaccharide; LBP, LPS-binding protein; MD2, myeloid differentiation protein 2; MyD88, myeloid differentiation primary response gene 88; TLR4, Toll-like receptor 4; TIRAP, Toll/IL-1 receptor domain containing adaptor protein; IRAK, IL-1 receptor-associated kinase; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-related kinases; HMGB1, high-mobility group box protein 1; RAGE, The receptor for advanced glycation end product; TGF-β, transforming growth factor beta; TβR, TGF-beta receptor; TAK1, TGF-β-activated kinase 1.
5.1.1. MAPK signaling pathway
The MAPK signaling pathway has been widely studied and is generally divided into four branches: the extracellular signal-related kinase (ERK1/2), p38 MAPK, c-Jun N-terminal kinase (JNK1/2/3), and ERK5125. Recent studies have reported that the activated MAPK signaling pathway may play a role in the pathogenesis of fibrosis126, 127, 128. Moreover, it was reported that activation of the MAPK signaling pathway in proinflammatory M1 macrophages was related to the production of the profibrotic mediators IL-1β, IL-6 and TNF-α129,130, which stimulate fibroblast activation and survival, thus, exacerbating fibrosis131. Interestingly, Liu et al.132 found that PGRN significantly inhibited LPS-activated JNK and p38 to complete its reversal of LPS-promoted M1 polarization. Based on these findings, it was reasonable to deduce that the activation of MAPK signaling pathways might be a potential mechanism of fibrosis. Similarly, in a study on ALI, PGRN suppressed the phosphorylation of ERK, JNK and p38 in the process of alleviating ALI102. In addition, Liu et al.17 demonstrated that high-mobility group box protein 1 (HMGB1) significantly exacerbates fibrosis formation in house dust mite-induced chronic asthma, while the upregulation of HMGB1 and subsequent RAGE/MAPKs signaling activation are restrained in PGRN-treated asthmatic mice. Overall, the mechanisms linking PGRN with fibrotic diseases are not fully understood, but given such findings, it is conceivable that PGRN might attenuate fibrosis by modulating MAPK signaling.
5.1.2. TGF-β/Smad signaling pathway
The TGF-β family of cytokines, which consists of TGF-βs, bone morphogenic proteins, and activins, regulates a wide array of biological activities in various cell types and at different developmental stages133. In general, canonical intracellular signaling activated by TGF-β ligands is mediated by SMAD family proteins, which are divided into three groups: receptor-associated SMADs (R-SMADs), cooperating SMADs (Co-SMADs), and inhibitory SMADs (ISMADs)134. However, a large amount of previous research into the signaling pathway has concentrated on its effect on fibrogenesis135,136. For example, a reduction in PGRN was shown to considerably increase the extent of fibrosis and collagen I accompanied by the upregulation of the expression of TGF-β1, Smad3 and P-Smad3, indicating that PGRN likely inhibited the degree of fibrosis via the TGF-β/Smad signaling pathway11. However, Zhou et al.137 found that PGRN induced TGF-β1 and p-SMAD2&3 protein expression in fibroblasts, and the stimulatory effect of PGRN on fibroblasts could be subsequently suppressed by a TGF-β signaling pathway inhibitor (HY-10431), as proven by the decreases in the α-SMA and collagen I and III levels. Interestingly, these findings are consistent with those of a previous investigation, which revealed that PGRN promoted the development of dermal fibrosis through the activation of TGF-β/Smad3 signaling111. Considering that different fibrotic diseases have complex molecular mechanisms, PGRN may play disparate roles, even if it only modulates the TGF-β/Smad signaling pathway.
5.1.3. NF-κB signaling pathway
The NF-κB signaling pathway is a highly conserved evolutionary pathway, activated by various stimuli that potentially endanger the host and has key functions in the initiation of immune, inflammatory and wound-healing responses62. Furthermore, there is an ever-increasing body of research evidence demonstrating that NF-κB signaling is a promising therapeutic method for treating various fibrotic diseases138,139. Based on a typical study on fibrosis investigated by Yoo et al.16, it has been shown that the phosphorylation of NF-κB was strongly decreased in PGRN-treated fibrosis mouse models compared with control groups, which suggested that PGRN reduces hepatic fibrosis by inhibiting inflammation activated by the NF-κB signaling pathway. Coincidentally, Liu et al.132 also illustrated the anti-inflammatory role of PGRN through the repression of NF-κB signaling. Given the complexity of the NF-κB signaling pathway, further comprehensive research is needed to assess the interaction between PGRN and NF-κB signaling in regulating fibrotic diseases.
5.2. PGRN/TNFR interactions
As we all know, no biologically active ligand functions in isolation but acts through its interaction with binding partners. There is no direct evidence that PGRN and TNFR binding modulate the fibrotic process. However, inflammation is believed to be an important mechanism in the development of fibrosis140. The importance and validity of PGRN-TNFR interactions in inflammatory diseases and conditions are supported by more recent experimental and epidemiological evidence42,45,141. There are two distinct TNFRs, TNFR1 and TNFR2142, which bind TNF-with comparable affinities but have different expression patterns and mediate different intracellular pathways143,144. After TNF binds to TNFR1, several adaptor proteins are recruited, followed by the activation of inflammatory signaling pathways145. In contrast, TNFR2 signaling is thought to mediate anti-inflammatory responses. In an LPS-induced acute lung injury model, PGRN markedly reversed LPS-induced lung permeability, as assessed by reductions in total protein, albumin, and IgM in BALF, as well as body weight loss106. This function is mediated through TNFR2, since neutralizing an antibody against TNFR2, but not TNFR1, completely blocks the therapeutic function of PGRN106. Furthermore, TNF-α and IL-β can induce NF-κB signaling in HSCs, promoting their survival and differentiation into myofibroblasts146. Consistent with this, TNFR1-deficient mice exhibit decreased liver fibrosis in response to bile duct ligation147. This is consistent with the data found by Sundaram et al.148. They revealed a mechanism to protect against liver fibrosis via the inhibition of TNFR signaling. Apparently, the interaction of PGRN with TNFR may modulate the fibrotic process. Although PGRN clearly has anti-inflammatory and antifibrotic effects on several diseases, it is unclear how PGRN exerts these effects. The finding that PGRN directly binds to TNFR and blocks the binding of TNF-α to its receptors provides new insight into the molecular mechanisms underlying PGRN-mediated antifibrosis.
6. Potential clinical significance of PGRN
6.1. PGRN as a biomarker for fibrosis
Fibrosis in the early stage is typically clinically reticent, and by the time patients reach clinical attention, fibrotic organs usually progress to a degree in which the physiologic functions of the affected tissue are impaired149. However, there is a problem that until now, no adequately exact biomarkers displaying the activity or condition of the fibrotic process have been identified. Hence, specific biomarkers are urgently needed to immediately diagnose fibrotic disorders, enhance clinical treatment, and expedite the advancement of effective therapeutic approaches150. Furthermore, specific biomarkers could accurately stratify fibrosis severity in high-risk individuals, closely reflect fibrosis progression, and could be utilized to establish customized treatments most appropriate for a specific subpopulation of fibrosis patients151.
Increased expression of serum PGRN has been detected in a variety of fibrotic illnesses and its benefit as a prognostic biomarker for dissimilar clinical parameters has been broadly investigated in many cohorts (as concluded above). It is likely that a single biomarker can predict the prognosis of fibrosis. Thus, the use of a group of biomarkers could increase the accuracy of fibrosis diagnosis and incorporation of PGRN may provide more vital information in diagnosis. A case in point is that PGRN together with activin A, which are possible markers, increase the accuracy of assessing the activity of the AE-IPF12. It has been shown that in stable IPF, PGRN levels did not differ from healthy controls88, while the expression level of activin A was elevated152. In AE-IPF, PGRN level was significantly higher than that in stable IPF and much higher than activin A expression (83.7 ± 10.0 vs. 14.2 ± 1.7 ng/mL)12. It is conceivable that this biomarker could be more extensively applied, for example, in the appraisal of cardiac remodeling after MI65. Importantly, serum PGRN was found to function as an independent marker of liver fibrosis in NAFLD15. In addition, decreased serum PGRN has been validated to be correlated with clinical disorder severity and increased liver stiffness in patients with GD69.
Taken together, these findings indicate that the application of PGRN as a proxy for successful treatment is promising but supplementary studies are needed to confirm these findings and to assess its use as a biomarker for the efficacy of experimental antifibrotic therapies.
6.2. PGRN as a therapeutic target in fibrotic diseases
A large number of clinical trials on PGRN have been registered worldwide in the treatment of frontotemporal dementia, advanced solid tumors, etc. (Table 2)153,154. Moreover, PGRN itself may contribute to the pathology of fibrotic diseases and is therefore a beneficial therapeutic target. For example, several investigations have noted that PGRN protects against fibrogenesis by inhibiting several profibrotic signaling pathways, which might provide new insights into the treatment of fibrotic diseases11,17. Moreover, follow-up studies have shown that PGRN and TNF-α can bind to the same TNFRs45. More importantly, compared to TNF-α, PGRN exhibited an even greater binding affinity for TNFRs. Based on these findings, Atsttrin, an engineered protein derived from PGRN42, was utilized to inhibit subchondral sclerosis which was worsened by the upregulation of MMP-1327. Moreover, it has been proved that PGRN decreased MMP-13 expression by inhibiting the binding of TNF-α to its receptor155. In another study on fibrosis, blocking MMP-13 also had potential therapeutic implications for preventing liver fibrosis156. As a result, the usage of PGRN can serve as a novel approach for targeting fibrosis.
Table 2.
Current, known clinical trials about PGRN in diseases.
| Disease | Subject | Targeting strategy | Result | Ref./clinical trial name |
|---|---|---|---|---|
| Frontotemporal dementia (FTD) | Patients in a phase 1 clinical trial | Compound enhancing PGRN expression, nimodipine | Unavailable | NCT01835665 |
| Patients in a phase 1/2 clinical trial | Compound enhancing PGRN expression, LY3884963 | Recruiting | NCT04408625 | |
| Patients in a phase 1/2 clinical trial | Compound enhancing PGRN expression, PBFT02 | Recruiting | NCT04747431 | |
| Patients in a phase 1/2 clinical trial | Compound enhancing PGRN expression, AAV:PGRN (AVB-101) | Recruiting | NCT06064890 | |
| Patients in a phase 2 clinical trial | Compound enhancing PGRN expression, FRM-0334 | Unavailable | NCT02149160 | |
| Patients in a phase 3 clinical trial | Compound enhancing PGRN expression, AL001 | Unavailable | NCT04374136 | |
| Patients in a phase 3 clinical trial | Compound enhancing PGRN expression, PI-2620 | Recruiting | NCT05456503 | |
| Advanced solid tumors | Patients in a phase 1 clinical trial | Binding to human PGRN/GP88, AG01 (an anti-PGRN/GP88 antibody) | Recruiting | NCT05627960 |
| Sepsis | Patients with sepsis | NA | Upregulated during the early stages of sepsis; a biomarker for sepsis | NCT03280576, Ref. 153 |
| Breast cancer | Eligible healthy women ≥40 years old at average risk for developing breast cancer | NA | Unavailable | NCT02700776 |
| Metabolic Syndrome | Patients with metabolic syndrome healthy subjects | NA | An independent predictor for atherosclerosis in subjects without metabolic syndrome | NCT01668888, Ref. 154 |
| NA | Unknown status | NCT04451616 |
FTD: frontotemporal dementia, PGRN: progranulin, AAV: adeno-associated virus.
NA, the targeting strategy for PGRN is not mentioned in the clinical trial data.
Another potential method to target PGRN is by applying microRNAs (miRNAs). It has been demonstrated that miRNAs are involved in multiple disease processes through targeting PGRN157,158. This finding indicates the occurrence of a negative feedback mechanism limiting the production of PGRN following some cytokine stimulation. As the underlying mechanisms will be explored in greater depth, such a negative feedback mechanism could conceivably be lost or enhanced in fibroblasts from patients with fibrosis. However, the translation of miRNA-based therapeutics into the clinic has been hampered by issues associated with specificity and delivery. A limitation is that the approach of treatment targeting PGRN can have undesired off-target effects because of a miRNA downstream effect on multiple genes. Other issues are related to delivery: the instability of ‘naked’, chemically unmodified miRNA structures and the lack of suitable delivery vehicles159. Thus, reorganizing these miRNAs will be another method to suppress or increase PGRN enrichment with possible therapeutic benefit.
Collectively, PGRN can directly bind to TNFR or indirectly be targeted by miRNAs to affect the occurrence and progression of fibrotic diseases.
7. Conclusion and future perspectives
PGRN expression is usually elevated when fibrosis develops in organs, including the liver, lung, skin and heart. Considering the abnormal expression of PGRN in patients with fibrotic diseases and its clinical significance in the process of this disease, PGRN could be a biomarker for identifying patients with fibrotic diseases and evaluating the severity and procession of this disease. Notably, overexpression of PGRN is likely to alleviate fibrotic processes by limiting the biological functions (proinflammatory and profibrotic) of PGRN targets, which validates the therapeutic potential of modulating PGRN.
However, many unresolved questions about the role of PGRN in fibrotic diseases remain to be further explored. Serum PGRN level is usually low, being up-regulated in the inflammatory state160. It has been demonstrated that PGRN was involved in chronic subclinical inflammation associated with the pathogenesis of diabetic microangiopathy and correlated with changes in disease metrics over time161. From the above, inflammation is an important mechanism for the occurrence of fibrosis. Interestingly, it remains to be investigated whether PGRN can be a biomarker for fibrosis progression and prognosis. Within the central nervous system, insights into the processing of PGRN into its granulin cleavage products in the lysosome have been relatively recent, but much remains to be learned about the interplay between PGRN and granulins, as well as the contribution of PGRN to fibrosis. As described above, several studies have reported the abnormal expression of PGRN in fibrotic diseases, but the outcomes are contradictory and inconclusive. For example, additional efforts are warranted to elucidate the dual role of PGRN in skin fibrosis. Mechanistically, in addition to acting on the TGF-β/Smad, MAPK and NF-κB signaling pathways, PGRN has been shown to be involved in the modulation of the Wnt/β-catenin signaling pathway, which affects inflammatory functions162. However, whether Wnt/β-catenin and PGRN are directly or indirectly related to fibrosis has yet to be determined. Given that TNF signaling is known to be involved in various kinds of diseases, and TNF inhibitors have been used to treat several kinds of inflammatory diseases, including osteoarthritis and skin inflammation, we envisage potential applications of PGRN, especially its derivative Atsttrin, in fibrotic diseases, such as skin fibrosis. As TNF inhibitors, PGRN and Atsttrin selectively target TNFR, and it is worthwhile to determine whether blockage of both ligand and receptors simultaneously will be more effective through comparing the effects of Atsttrin alone or with current TNF inhibitors. Finally, a crucial point is represented by the timing of the therapeutic interventions. A growing body of evidence suggests that fibrosis occurs in the early stage and is accompanied by inflammation. Therefore, ideally, treatment should be administered not only to patients but also to high-risk people.
In conclusion, notwithstanding these urgent problems, the available evidence strongly supports that PGRN may be a novel biomarker and therapeutic target for reversing the course of multiple fibrotic diseases. More in-depth mechanistic studies are still needed to validate the clinical relevance of PGRN and the potential for therapeutic effectiveness of PGRN in fibrotic diseases.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Nos. 81274172, 81473267, 81973637 and 82373933); The National Traditional Chinese Medicine Inheritance and Innovation “Hundreds and Thousands” Talent Project: Young Qihuang Scholar Support Project of the State Administration of Traditional Chinese Medicine in 2020 (China).
Author contributions
Fan Yang: Writing – original draft. Ming-Han Cheng: Writing – review & editing. Hai-Feng Pan: Conceptualization. Jian Gao: Conceptualization, Supervision.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Hai-Feng Pan, Email: panhaifeng1982@sina.com.
Jian Gao, Email: gaojianayfy@163.com.
References
- 1.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson N.C., Rieder F., Wynn T.A. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piera-Velazquez S., Mendoza F.A., Jimenez S.A. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med. 2016;5:45. doi: 10.3390/jcm5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang D.Q., Terrault N.A., Tacke F., Gluud L.L., Arrese M., Bugianesi E., et al. Global epidemiology of cirrhosis—aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388–398. doi: 10.1038/s41575-023-00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch JP 3rd, Huynh R.H., Fishbein M.C., Saggar R., Belperio J.A., Weigt S.S. Idiopathic pulmonary fibrosis: epidemiology, clinical features, prognosis, and management. Semin Respir Crit Care Med. 2016;37:331–357. doi: 10.1055/s-0036-1582011. [DOI] [PubMed] [Google Scholar]
- 6.Shi N., Wang Z., Zhu H., Liu W., Zhao M., Jiang X., et al. Research progress on drugs targeting the TGF-beta signaling pathway in fibrotic diseases. Immunol Res. 2022;70:276–288. doi: 10.1007/s12026-022-09267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X., Kwan J.Y.Y., Yip K., Liu P.P., Liu F.F. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19:57–75. doi: 10.1038/s41573-019-0040-5. [DOI] [PubMed] [Google Scholar]
- 8.Murtha L.A., Morten M., Schuliga M.J., Mabotuwana N.S., Hardy S.A., Waters D.W., et al. The role of pathological aging in cardiac and pulmonary fibrosis. Aging Dis. 2019;10:419–428. doi: 10.14336/AD.2018.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardali E., Sanchez-Duffhues G., Gomez-Puerto M.C., Ten Dijke P. TGF-beta-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci. 2017;18:2157. doi: 10.3390/ijms18102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X., Chen J., Sun H., Zhang Y., Zou D. New insights into fibrosis from the ECM degradation perspective: the macrophage–MMP–ECM interaction. Cell Biosci. 2022;12:117. doi: 10.1186/s13578-022-00856-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S.S., Zhang M.X., Wang Y., Wang W., Zhao C.M., Sun X.M., et al. Reduction of PGRN increased fibrosis during skin wound healing in mice. Histol Histopathol. 2019;34:765–774. doi: 10.14670/HH-18-076. [DOI] [PubMed] [Google Scholar]
- 12.Xie T., Han L., Chen Y., Wu H. Progranulin and activin A concentrations are elevated in serum from patients with acute exacerbations of idiopathic pulmonary fibrosis. Lung. 2021;199:467–473. doi: 10.1007/s00408-021-00470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C., Zhang L., Ndong J.C., Hettinghouse A., Sun G., Chen C., et al. Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J Neuroinflammation. 2019;16:238. doi: 10.1186/s12974-019-1630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogonowska M., Poniatowski L.A., Wawrzyniak A., Krolikowska K., Kalicki B. The role of progranulin (PGRN) in the modulation of anti-inflammatory response in asthma. Cent Eur J Immunol. 2019;44:97–101. doi: 10.5114/ceji.2019.83267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yilmaz Y., Eren F., Yonal O., Polat Z., Bacha M., Kurt R., et al. Serum progranulin as an independent marker of liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Dis Markers. 2011;31:205–210. doi: 10.3233/DMA-2011-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo W., Lee J., Noh K.H., Lee S., Jung D., Kabir M.H., et al. Progranulin attenuates liver fibrosis by downregulating the inflammatory response. Cell Death Dis. 2019;10:758. doi: 10.1038/s41419-019-1994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M., Shan M., Zhang Y., Guo Z. Progranulin protects against airway remodeling through the modulation of autophagy via HMGB1 suppression in house dust mite-induced chronic asthma. J Inflamm Res. 2021;14:3891–3904. doi: 10.2147/JIR.S322724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S., Liu J., Guo J., Xu Y., Zhou Z., Li Z., et al. Progranulin inhibits fibrosis by interacting with and up-regulating DNAJC3 during mouse skin wound healing. Cell Signal. 2023;109 doi: 10.1016/j.cellsig.2023.110770. [DOI] [PubMed] [Google Scholar]
- 19.Zanocco-Marani T., Bateman A., Romano G., Valentinis B., He Z.H., Baserga R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- 20.Baba T., Hoff H.B., 3rd, Nemoto H., Lee H., Orth J., Arai Y., et al. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev. 1993;34:233–243. doi: 10.1002/mrd.1080340302. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J., Gao G., Crabb J.W., Serrero G. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J Biol Chem. 1993;268:10863–10869. [PubMed] [Google Scholar]
- 22.Tanimoto R., Lu K.G., Xu S.Q., Buraschi S., Belfiore A., Iozzo R.V., et al. Mechanisms of progranulin action and regulation in genitourinary cancers. Front Endocrinol. 2016;7:100. doi: 10.3389/fendo.2016.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams A., Wang E.C., Thurner L., Liu C.J. Review: novel insights into tumor necrosis factor receptor, death receptor 3, and progranulin pathways in arthritis and bone remodeling. Arthritis Rheumatol. 2016;68:2845–2856. doi: 10.1002/art.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hrabal R., Chen Z., James S., Bennett H.P., Ni F. The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nat Struct Biol. 1996;3:747–752. doi: 10.1038/nsb0996-747. [DOI] [PubMed] [Google Scholar]
- 25.Rhinn H., Tatton N., McCaughey S., Kurnellas M., Rosenthal A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol Sci. 2022;43:641–652. doi: 10.1016/j.tips.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Fu W., Hu W., Yi Y.S., Hettinghouse A., Sun G., Bi Y., et al. TNFR2/14-3-3epsilon signaling complex instructs macrophage plasticity in inflammation and autoimmunity. J Clin Invest. 2021;131 doi: 10.1172/JCI144016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu W., Hettinghouse A., Chen Y., Hu W., Ding X., Chen M., et al. 14-3-3 epsilon is an intracellular component of TNFR2 receptor complex and its activation protects against osteoarthritis. Ann Rheum Dis. 2021;80:1615–1627. doi: 10.1136/annrheumdis-2021-220000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid A., Hochberg A., Kreiss A.F., Gehl J., Patz M., Thomalla M., et al. Role of progranulin in adipose tissue innate immunity. Cytokine. 2020;125 doi: 10.1016/j.cyto.2019.154796. [DOI] [PubMed] [Google Scholar]
- 29.Yabe K., Yamamoto Y., Takemura M., Hara T., Tsurumi H., Serrero G., et al. Progranulin depletion inhibits proliferation via the transforming growth factor beta/SMAD family member 2 signaling axis in Kasumi-1 cells. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2020.e05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang W., Zhou T., Shi H., Yao M., Zhang D., Qian H., et al. Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion. J Exp Clin Cancer Res. 2021;40:4. doi: 10.1186/s13046-020-01786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia X., Serrero G. Identification of cell surface binding sites for PC-cell-derived growth factor, PCDGF, (epithelin/granulin precursor) on epithelial cells and fibroblasts. Biochem Biophys Res Commun. 1998;245:539–543. doi: 10.1006/bbrc.1998.8498. [DOI] [PubMed] [Google Scholar]
- 32.Wang C., Zhou W., Su G., Hu J., Yang P. Progranulin suppressed autoimmune uveitis and autoimmune neuroinflammation by inhibiting Th1/Th17 cells and promoting treg cells and M2 macrophages. Neurol Neuroimmunol Neuroinflamm. 2022;9 doi: 10.1212/NXI.0000000000001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., He Q., Zhang X., Ma Y., Fan F., Dong Y., et al. Innate anti-microbial and anti-chemotaxis properties of progranulin in an acute otitis media mouse model. Front Immunol. 2018;9:2952. doi: 10.3389/fimmu.2018.02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadagavadi R.K., Reeves W.B. NODding off in acute kidney injury with progranulin? Kidney Int. 2015;87:873–875. doi: 10.1038/ki.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez E.M., Mongiat M., Slater S.J., Baffa R., Iozzo R.V. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. J Biol Chem. 2003;278:38113–38116. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J., Nathan C., Jin W., Sim D., Ashcroft G.S., Wahl S.M., et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 37.Okura H., Yamashita S., Ohama T., Saga A., Yamamoto-Kakuta A., Hamada Y., et al. HDL/apolipoprotein A-I binds to macrophage-derived progranulin and suppresses its conversion into proinflammatory granulins. J Atherosclerosis Thromb. 2010;17:568–577. doi: 10.5551/jat.3921. [DOI] [PubMed] [Google Scholar]
- 38.Sui D., Wilson J.E. Interaction of insulin-like growth factor binding protein-4, Miz-1, leptin, lipocalin-type prostaglandin D synthase, and granulin precursor with the N-terminal half of type III hexokinase. Arch Biochem Biophys. 2000;382:262–274. doi: 10.1006/abbi.2000.2019. [DOI] [PubMed] [Google Scholar]
- 39.Lam C.Y., Yip C.W., Poon T.C., Cheng C.K., Ng E.W., Wong N.C., et al. Identification and characterization of tropomyosin 3 associated with granulin-epithelin precursor in human hepatocellular carcinoma. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida S., Zhou L., Gao F.B. Progranulin, a glycoprotein deficient in frontotemporal dementia, is a novel substrate of several protein disulfide isomerase family proteins. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo F., Lai Y., Tian Q., Lin E.A., Kong L., Liu C. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62:2023–2036. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian Q., Zhao Y., Mundra J.J., Gonzalez-Gugel E., Jian J., Uddin S.M., et al. Three TNFR-binding domains of PGRN act independently in inhibition of TNF-alpha binding and activity. Front Biosci. 2014;19:1176–1185. doi: 10.2741/4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyakawa S., Sakuma H., Warude D., Asanuma S., Arimura N., Yoshihara T., et al. Anti-sortilin1 antibody up-regulates progranulin via Sortilin1 down-regulation. Front Neurosci. 2020;14 doi: 10.3389/fnins.2020.586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moresco E.M., Beutler B. Special delivery: granulin brings CpG DNA to Toll-like receptor 9. Immunity. 2011;34:453–455. doi: 10.1016/j.immuni.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang W., Lu Y., Tian Q.Y., Zhang Y., Guo F.J., Liu G.Y., et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voshtani R., Song M., Wang H., Li X., Zhang W., Tavallaie M.S., et al. Progranulin promotes melanoma progression by inhibiting natural killer cell recruitment to the tumor microenvironment. Cancer Lett. 2019;465:24–35. doi: 10.1016/j.canlet.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu D., Suenaga N., Edelmann M.J., Fridman R., Muschel R.J., Kessler B.M. Novel MMP-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol Cell Proteomics. 2008;7:2215–2228. doi: 10.1074/mcp.M800095-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kessenbrock K., Frohlich L., Sixt M., Lammermann T., Pfister H., Bateman A., et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118:2438–2447. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suh H.S., Choi N., Tarassishin L., Lee S.C. Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12) PLoS One. 2012;7 doi: 10.1371/journal.pone.0035115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Van Damme P., Cruchaga C., Gitcho M.A., Vidal J.M., Seijo-Martinez M., et al. Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J Neurochem. 2010;112:1305–1315. doi: 10.1111/j.1471-4159.2009.06546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunha P.S., Laranjo S., Heijman J., Oliveira M.M. The atrium in atrial fibrillation—a clinical review on how to manage atrial fibrotic substrates. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.879984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao M., Wang L., Wang M., Zhou S., Lu Y., Cui H., et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct Targeted Ther. 2022;7:206. doi: 10.1038/s41392-022-01070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee H.N., Na H.K., Surh Y.J. Resolution of inflammation as a novel chemopreventive strategy. Semin Immunopathol. 2013;35:151–161. doi: 10.1007/s00281-013-0363-y. [DOI] [PubMed] [Google Scholar]
- 54.Xu C.G., Zhu X.L., Wang W., Zhou X.J. Ursolic acid inhibits epithelial-mesenchymal transition in vitro and in vivo. Pharm Biol. 2019;57:169–175. doi: 10.1080/13880209.2019.1577464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mascharak S., Talbott H.E., Januszyk M., Griffin M., Chen K., Davitt M.F., et al. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell. 2022;29:315–327. doi: 10.1016/j.stem.2021.12.011. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinz B., Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16:11–31. doi: 10.1038/s41584-019-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X., Kukar T., Rademakers R. Lysosomal dysfunction and other pathomechanisms in ftld: evidence from progranulin genetics and biology. Adv Exp Med Biol. 2021;1281:219–242. doi: 10.1007/978-3-030-51140-1_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang B.C., Liu H., Talwar A., Jian J. New discovery rarely runs smooth: an update on progranulin/TNFR interactions. Protein Cell. 2015;6:792–803. doi: 10.1007/s13238-015-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wen Y., Lu X., Ren J., Privratsky J.R., Yang B., Rudemiller N.P., et al. KLF4 in macrophages attenuates TNFalpha-mediated kidney injury and fibrosis. J Am Soc Nephrol. 2019;30:1925–1938. doi: 10.1681/ASN.2019020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Therrien F.J., Agharazii M., Lebel M., Lariviere R. Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am J Nephrol. 2012;36:151–161. doi: 10.1159/000340033. [DOI] [PubMed] [Google Scholar]
- 61.Osawa Y., Hoshi M., Yasuda I., Saibara T., Moriwaki H., Kozawa O. Tumor necrosis factor-alpha promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luedde T., Schwabe R.F. NF-kappaB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tschumperlin D.J., Lagares D. Mechano-therapeutics: targeting mechanical signaling in fibrosis and tumor stroma. Pharmacol Ther. 2020;212 doi: 10.1016/j.pharmthera.2020.107575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang G., An L., Fan M., Zhang M., Chen B., Zhu M., et al. Potential role of full-length and nonfull-length progranulin in affecting aortic valve calcification. J Mol Cell Cardiol. 2020;141:93–104. doi: 10.1016/j.yjmcc.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Sasaki T., Shimazawa M., Kanamori H., Yamada Y., Nishinaka A., Kuse Y., et al. Effects of progranulin on the pathological conditions in experimental myocardial infarction model. Sci Rep. 2020;10 doi: 10.1038/s41598-020-68804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernandez-Gea V., Friedman S.L. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 68.Parola M., Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspect Med. 2019;65:37–55. doi: 10.1016/j.mam.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Tantawy A.A., Adly A.A., Ismail E.A., Salah N.Y., Abdel Alem S., Elsantiel H.I. Serum progranulin levels in paediatric patients with Gaucher disease; relation to disease severity and liver stiffness by transient elastography. Liver Int. 2020;40:3051–3060. doi: 10.1111/liv.14598. [DOI] [PubMed] [Google Scholar]
- 70.Afinogenova Y., Ruan J., Yang R., Kleytman N., Pastores G., Lischuk A., et al. Aberrant progranulin, YKL-40, cathepsin D and cathepsin S in Gaucher disease. Mol Genet Metabol. 2019;128:62–67. doi: 10.1016/j.ymgme.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guerra R.R., Trotta M.R., Parra O.M., Avanzo J.L., Bateman A., Aloia T.P., et al. Modulation of extracellular matrix by nutritional hepatotrophic factors in thioacetamide-induced liver cirrhosis in the rat. Braz J Med Biol Res. 2009;42:1027–1034. doi: 10.1590/s0100-879x2009005000027. [DOI] [PubMed] [Google Scholar]
- 73.Mathieu P., Boulanger M.C. Basic mechanisms of calcific aortic valve disease. Can J Cardiol. 2014;30:982–993. doi: 10.1016/j.cjca.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 74.Kraler S., Blaser M.C., Aikawa E., Camici G.G., Luscher T.F. Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J. 2022;43:683–697. doi: 10.1093/eurheartj/ehab757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Muynck L., Van Damme P. Cellular effects of progranulin in health and disease. J Mol Neurosci. 2011;45:549–560. doi: 10.1007/s12031-011-9553-z. [DOI] [PubMed] [Google Scholar]
- 76.Piscopo P., Rivabene R., Adduci A., Mallozzi C., Malvezzi-Campeggi L., Crestini A., et al. Hypoxia induces up-regulation of progranulin in neuroblastoma cell lines. Neurochem Int. 2010;57:893–898. doi: 10.1016/j.neuint.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Olczak M., Poniatowski L.A., Siwinska A., Kwiatkowska M., Chutoranski D., Wierzba-Bobrowicz T. Elevated serum and urine levels of progranulin (PGRN) as a predictor of microglia activation in the early phase of traumatic brain injury: a further link with the development of neurodegenerative diseases. Folia Neuropathol. 2021;59:81–90. doi: 10.5114/fn.2021.105137. [DOI] [PubMed] [Google Scholar]
- 78.Yin F., Banerjee R., Thomas B., Zhou P., Qian L., Jia T., et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 80.Lu L., Liu M., Sun R., Zheng Y., Zhang P. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72:865–867. doi: 10.1007/s12013-015-0553-4. [DOI] [PubMed] [Google Scholar]
- 81.Hume R.D., Chong J.J.H. The Cardiac injury immune response as a target for regenerative and cellular therapies. Clin Therapeut. 2020;42:1923–1943. doi: 10.1016/j.clinthera.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 82.Jiao J., He S., Wang Y., Lu Y., Gu M., Li D., et al. Regulatory B cells improve ventricular remodeling after myocardial infarction by modulating monocyte migration. Basic Res Cardiol. 2021;116:46. doi: 10.1007/s00395-021-00886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meloni M., Caporali A., Graiani G., Lagrasta C., Katare R., Van Linthout S., et al. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res. 2010;106:1275–1284. doi: 10.1161/CIRCRESAHA.109.210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spagnolo P., Kropski J.A., Jones M.G., Lee J.S., Rossi G., Karampitsakos T., et al. Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol Ther. 2021;222 doi: 10.1016/j.pharmthera.2020.107798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kreuter M., Maher T.M. Treatment of acute exacerbation of idiopathic pulmonary fibrosis. a call to arms. Am J Respir Crit Care Med. 2020;201:1030–1032. doi: 10.1164/rccm.202001-0057ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinez F.J., Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:315–321. doi: 10.1513/pats.200602-022TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toth N.M., Muller V., Nagy T., Polivka L., Horvath P., Bohacs A., et al. Serum progranulin level might differentiate non-IPF ILD from IPF. Int J Mol Sci. 2023;24:9178. doi: 10.3390/ijms24119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeng Z., Huang H., Zhang J., Liu Y., Zhong W., Chen W., et al. HDM induce airway epithelial cell ferroptosis and promote inflammation by activating ferritinophagy in asthma. Faseb J. 2022;36 doi: 10.1096/fj.202101977RR. [DOI] [PubMed] [Google Scholar]
- 90.Papi A., Brightling C., Pedersen S.E., Reddel H.K. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 91.Pascoe C.D., Green F.H.Y., Elliot J.G., James A.L., Noble P.B., Donovan G.M. Airway remodelling with spatial correlations: implications for asthma pathogenesis. Respir Physiol Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103469. [DOI] [PubMed] [Google Scholar]
- 92.Poon A.H., Choy D.F., Chouiali F., Ramakrishnan R.K., Mahboub B., Audusseau S., et al. Increased autophagy-related 5 gene expression is associated with collagen expression in the airways of refractory asthmatics. Front Immunol. 2017;8:355. doi: 10.3389/fimmu.2017.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li B.B., Chen Y.L., Pang F. MicroRNA-30a targets ATG5 and attenuates airway fibrosis in asthma by suppressing autophagy. Inflammation. 2020;43:44–53. doi: 10.1007/s10753-019-01076-0. [DOI] [PubMed] [Google Scholar]
- 94.Redington A.E. Fibrosis and airway remodelling. Clin Exp Allergy. 2000;30(Suppl 1):42–45. doi: 10.1046/j.1365-2222.2000.00096.x. [DOI] [PubMed] [Google Scholar]
- 95.Xiao K., He W., Guan W., Hou F., Yan P., Xu J., et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-kappaB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11:863. doi: 10.1038/s41419-020-03034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Butt Y., Kurdowska A., Allen T.C. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 97.Zoulikha M., Xiao Q., Boafo G.F., Sallam M.A., Chen Z., He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm Sin B. 2022;12:600–620. doi: 10.1016/j.apsb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kral J.B., Kuttke M., Schrottmaier W.C., Birnecker B., Warszawska J., Wernig C., et al. Sustained PI3K activation exacerbates BLM-induced lung fibrosis via activation of pro-inflammatory and pro-fibrotic pathways. Sci Rep. 2016;6 doi: 10.1038/srep23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng Z., Xiang M., Guan H., Liu Y., Zhang H., Xia L., et al. Early fibroproliferative signs on high-resolution CT are associated with mortality in COVID-19 pneumonia patients with ARDS: a retrospective study. Ther Adv Chronic Dis. 2021;12 doi: 10.1177/2040622320982171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marshall R.P., Bellingan G., Webb S., Puddicombe A., Goldsack N., McAnulty R.J., et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162:1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 101.Zheng F., Wu X., Zhang J., Fu Z., Zhang Y. Sevoflurane reduces lipopolysaccharide-induced apoptosis and pulmonary fibrosis in the RAW264.7 cells and mice models to ameliorate acute lung injury by eliminating oxidative damages. Redox Rep. 2022;27:139–149. doi: 10.1080/13510002.2022.2096339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu J., Huang J., Shan M., Hu X., Guo W., Xie W., et al. Progranulin ameliorates lung inflammation in an LPS-induced acute lung injury mouse model by modulating macrophage polarization and the MAPK pathway. Ann Clin Lab Sci. 2021;51:220–230. [PubMed] [Google Scholar]
- 103.Zhao Y.P., Wei J.L., Tian Q.Y., Liu A.T., Yi Y.S., Einhorn T.A., et al. Progranulin suppresses titanium particle induced inflammatory osteolysis by targeting TNFalpha signaling. Sci Rep. 2016;6 doi: 10.1038/srep20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eming S.A., Wynn T.A., Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 105.Chen Y.Q., Wang C.J., Xie K., Lei M., Chai Y.S., Xu F., et al. Progranulin improves acute lung injury through regulating the differentiation of regulatory T cells and interleukin-10 immunomodulation to promote macrophage polarization. Mediat Inflamm. 2020;2020 doi: 10.1155/2020/9704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo Z., Li Q., Han Y., Liang Y., Xu Z., Ren T. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediat Inflamm. 2012;2012 doi: 10.1155/2012/540794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gu Y., Han J., Jiang C., Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. 2020;59 doi: 10.1016/j.arr.2020.101036. [DOI] [PubMed] [Google Scholar]
- 108.Hu M.S., Borrelli M.R., Lorenz H.P., Longaker M.T., Wan D.C. Mesenchymal stromal cells and cutaneous wound healing: a comprehensive review of the background, role, and therapeutic potential. Stem Cell Int. 2018;2018 doi: 10.1155/2018/6901983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Talbott H.E., Mascharak S., Griffin M., Wan D.C., Longaker M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161–1180. doi: 10.1016/j.stem.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He Z., Ong C.H., Halper J., Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–229. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 111.Yang T., Zhang X., Chen A., Xiao Y., Sun S., Yan J., et al. Progranulin promotes bleomycin-induced skin sclerosis by enhancing transforming growth factor-beta/Smad3 signaling through up-regulation of transforming growth factor-beta type I receptor. Am J Pathol. 2019;189:1582–1593. doi: 10.1016/j.ajpath.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Denton C.P., Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 113.Bukiri H., Volkmann E.R. Current advances in the treatment of systemic sclerosis. Curr Opin Pharmacol. 2022;64 doi: 10.1016/j.coph.2022.102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meldrum K.K., Misseri R., Metcalfe P., Dinarello C.A., Hile K.L., Meldrum D.R. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1456–R1464. doi: 10.1152/ajpregu.00620.2005. [DOI] [PubMed] [Google Scholar]
- 115.Wen Y., Rudemiller N.P., Zhang J., Robinette T., Lu X., Ren J., et al. TNF-alpha in T lymphocytes attenuates renal injury and fibrosis during nephrotoxic nephritis. Am J Physiol Ren Physiol. 2020;318:F107–F116. doi: 10.1152/ajprenal.00347.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miyagawa T., Ichimura Y., Nakamura K., Hirabayashi M., Yamashita T., Saigusa R., et al. Progranulin overproduction due to constitutively activated c-Abl/PKC-δ/Fli1 pathway contributes to the resistance of dermal fibroblasts to the anti-fibrotic effect of tumor necrosis factor-α in localized scleroderma. J Dermatol Sci. 2018;92:207–214. doi: 10.1016/j.jdermsci.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Liu C., Li J., Shi W., Zhang L., Liu S., Lian Y., et al. Progranulin regulates inflammation and tumor. Antiinflamm Antiallergy Agents Med Chem. 2020;19:88–102. doi: 10.2174/1871523018666190724124214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tanaka A., Tsukamoto H., Mitoma H., Kiyohara C., Ueda N., Ayano M., et al. Serum progranulin levels are elevated in dermatomyositis patients with acute interstitial lung disease, predicting prognosis. Arthritis Res Ther. 2015;17:27. doi: 10.1186/s13075-015-0547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ungurs M.J., Sinden N.J., Stockley R.A. Progranulin is a substrate for neutrophil-elastase and proteinase-3 in the airway and its concentration correlates with mediators of airway inflammation in COPD. Am J Physiol Lung Cell Mol Physiol. 2014;306:L80–L87. doi: 10.1152/ajplung.00221.2013. [DOI] [PubMed] [Google Scholar]
- 120.Jing C., Zhang X., Song Z., Zheng Y., Yin Y. Progranulin mediates proinflammatory responses in systemic lupus erythematosus: implications for the pathogenesis of systemic lupus erythematosus. J Interferon Cytokine Res. 2020;40:33–42. doi: 10.1089/jir.2019.0047. [DOI] [PubMed] [Google Scholar]
- 121.Qiu F., Song L., Ding F., Liu H., Shu Q., Yang N., et al. Expression level of the growth factor progranulin is related with development of systemic lupus erythematosus. Diagn Pathol. 2013;8:88. doi: 10.1186/1746-1596-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xin P., Xu X., Deng C., Liu S., Wang Y., Zhou X., et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharm. 2020;80 doi: 10.1016/j.intimp.2020.106210. [DOI] [PubMed] [Google Scholar]
- 123.Ma S., Meng Z., Chen R., Guan K.L. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 124.Lin X., Farooqi A.A. Cucurbitacin mediated regulation of deregulated oncogenic signaling cascades and non-coding RNAs in different cancers: spotlight on JAK/STAT, Wnt/β-catenin, mTOR, TRAIL-mediated pathways. Semin Cancer Biol. 2021;73:302–309. doi: 10.1016/j.semcancer.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 125.Sun Y., Liu W.Z., Liu T., Feng X., Yang N., Zhou H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 126.Salloum S., Jeyarajan A.J., Kruger A.J., Holmes J.A., Shao T., Sojoodi M., et al. Fatty acids activate the transcriptional coactivator YAP1 to promote liver fibrosis via p38 mitogen-activated protein kinase. Cell Mol Gastroenterol Hepatol. 2021;12:1297–1310. doi: 10.1016/j.jcmgh.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li R., Guo Y., Zhang Y., Zhang X., Zhu L., Yan T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int J Mol Sci. 2019;20:1103. doi: 10.3390/ijms20051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang D., Deng B., Cheng L., Li J., Zhang J., Zhang X., et al. A novel and low-toxic peptide DR3penA alleviates pulmonary fibrosis by regulating the MAPK/miR-23b-5p/AQP5 signaling axis. Acta Pharm Sin B. 2023;13:722–738. doi: 10.1016/j.apsb.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goda C., Balli D., Black M., Milewski D., Le T., Ustiyan V., et al. Loss of FOXM1 in macrophages promotes pulmonary fibrosis by activating p38 MAPK signaling pathway. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X., Fan L., Wu J., Xu H., Leung W.Y., Fu K., et al. Macrophage p38α promotes nutritional steatohepatitis through M1 polarization. J Hepatol. 2019;71:163–174. doi: 10.1016/j.jhep.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 131.Zhang W.J., Chen S.J., Zhou S.C., Wu S.Z., Wang H. Inflammasomes and fibrosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.643149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu L., Guo H., Song A., Huang J., Zhang Y., Jin S., et al. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-κB and MAPK pathways. BMC Immunol. 2020;21:32. doi: 10.1186/s12865-020-00355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luo K. Signaling cross talk between TGF-beta/Smad and other signaling pathways. Cold Spring Harbor Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang J., Zhang X., Xie F., Zhang Z., van Dam H., Zhang L., et al. The regulation of TGF-β/SMAD signaling by protein deubiquitination. Protein Cell. 2014;5:503–517. doi: 10.1007/s13238-014-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang T., He X., Caldwell L., Goru S.K., Ulloa Severino L., Tolosa M.F., et al. NUAK1 promotes organ fibrosis via YAP and TGF-β/SMAD signaling. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.aaz4028. [DOI] [PubMed] [Google Scholar]
- 136.Yang Y., Sun M., Li W., Liu C., Jiang Z., Gu P., et al. Rebalancing TGF-β/Smad7 signaling via compound kushen injection in hepatic stellate cells protects against liver fibrosis and hepatocarcinogenesis. Clin Transl Med. 2021;11 doi: 10.1002/ctm2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou Y., Pang H., Wang J., Wu H., Xu Z., Liu X., et al. Progranulin promotes the formation and development of capsules caused by silicone in Sprague–Dawley rats. Clin Cosmet Invest Dermatol. 2022;15:1561–1573. doi: 10.2147/CCID.S374128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peng L., Wen L., Shi Q.F., Gao F., Huang B., Meng J., et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial–mesenchymal transition and inflammation. Cell Death Dis. 2020;11:978. doi: 10.1038/s41419-020-03178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y., Mou Q., Zhu Z., Zhao L., Zhu L. MALAT1 promotes liver fibrosis by sponging miR-181a and activating TLR4-NF-κB signaling. Int J Mol Med. 2021;48:215. doi: 10.3892/ijmm.2021.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang M., Haughey M., Wang N.Y., Blease K., Kapoun A.M., Couto S., et al. Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao Y.P., Tian Q.Y., Frenkel S., Liu C.J. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34:6412–6421. doi: 10.1016/j.biomaterials.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brenner D., Blaser H., Mak T.W. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362–374. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 143.Mundra J.J., Jian J., Bhagat P., Liu C.J. Progranulin inhibits expression and release of chemokines CXCL9 and CXCL10 in a TNFR1 dependent manner. Sci Rep. 2016;6 doi: 10.1038/srep21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wei F., Zhang Y., Jian J., Mundra J.J., Tian Q., Lin J., et al. PGRN protects against colitis progression in mice in an IL-10 and TNFR2 dependent manner. Sci Rep. 2014;4:7023. doi: 10.1038/srep07023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aggarwal B.B. Editorial: balancing tumor necrosis factor receptor I and tumor necrosis factor receptor II jointly for joint inflammation. Arthritis Rheumatol. 2014;66:2657–2660. doi: 10.1002/art.38753. [DOI] [PubMed] [Google Scholar]
- 146.Pradere J.P., Kluwe J., De Minicis S., Jiao J.J., Gwak G.Y., Dapito D.H., et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tarrats N., Moles A., Morales A., Garcia-Ruiz C., Fernandez-Checa J.C., Mari M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–327. doi: 10.1002/hep.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sundaram B., Behnke K., Belancic A., Al-Salihi M.A., Thabet Y., Polz R., et al. iRhom2 inhibits bile duct obstruction-induced liver fibrosis. Sci Signal. 2019;12 doi: 10.1126/scisignal.aax1194. [DOI] [PubMed] [Google Scholar]
- 149.Penke L.R., Peters-Golden M. Molecular determinants of mesenchymal cell activation in fibroproliferative diseases. Cell Mol Life Sci. 2019;76:4179–4201. doi: 10.1007/s00018-019-03212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marshall R.P., Simpson J.K., Lukey P.T. Strategies for biomarker discovery in fibrotic disease. Biochim Biophys Acta. 2013;1832:1079–1087. doi: 10.1016/j.bbadis.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 151.Hardy T., Zeybel M., Day C.P., Dipper C., Masson S., McPherson S., et al. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut. 2017;66:1321–1328. doi: 10.1136/gutjnl-2016-311526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Myllarniemi M., Tikkanen J., Hulmi J.J., Pasternack A., Sutinen E., Ronty M., et al. Upregulation of activin-B and follistatin in pulmonary fibrosis—a translational study using human biopsies and a specific inhibitor in mouse fibrosis models. BMC Pulm Med. 2014;14:170. doi: 10.1186/1471-2466-14-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brandes F., Borrmann M., Buschmann D., Meidert A.S., Reithmair M., Langkamp M., et al. Progranulin signaling in sepsis, community-acquired bacterial pneumonia and COVID-19: a comparative, observational study. Intensive Care Med Exp. 2021;9:43. doi: 10.1186/s40635-021-00406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yoo H.J., Hwang S.Y., Hong H.C., Choi H.Y., Yang S.J., Choi D.S., et al. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang S., Wei J., Fan Y., Ding H., Tian H., Zhou X., et al. Progranulin is positively associated with intervertebral disc degeneration by interaction with IL-10 and IL-17 through TNF pathways. Inflammation. 2018;41:1852–1863. doi: 10.1007/s10753-018-0828-1. [DOI] [PubMed] [Google Scholar]
- 156.George J., Tsutsumi M., Tsuchishima M. MMP-13 deletion decreases profibrogenic molecules and attenuates N-nitrosodimethylamine-induced liver injury and fibrosis in mice. J Cell Mol Med. 2017;21:3821–3835. doi: 10.1111/jcmm.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xie W., Lu Q., Wang K., Lu J., Gu X., Zhu D., et al. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J Cell Physiol. 2018;233:6615–6631. doi: 10.1002/jcp.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen L., Li Q., Wang J., Jin S., Zheng H., Lin J., et al. MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J Cell Mol Med. 2017;21:3347–3359. doi: 10.1111/jcmm.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics—challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu L., Zhou B., Li H., Liu J., Du J., Zang W., et al. Serum levels of progranulin are closely associated with microvascular complication in type 2 diabetes. Dis Markers. 2015;2015 doi: 10.1155/2015/357279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Albeltagy E.S., Hammour A.E., Albeltagy S.A. Potential value of serum progranulin as a biomarker for the presence and severity of micro vascular complications among Egyptian patients with type 2 diabetes mellitus. J Diabetes Metab Disord. 2019;18:217–228. doi: 10.1007/s40200-019-00406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tian R., Li Y., Yao X. PGRN suppresses inflammation and promotes autophagy in keratinocytes through the Wnt/beta-catenin signaling pathway. Inflammation. 2016;39:1387–1394. doi: 10.1007/s10753-016-0370-y. [DOI] [PubMed] [Google Scholar]