Abstract
Gp80, a cell-adhesion molecule in Dictyostelium discoideum, is modified by N- and O-linked oligosaccharides, and a glycosylphosphatidylinositol (GPI) anchor. To identify sequences important for the addition of these modifications to gp80, we created a hybrid protein in which the C-terminal 136 amino acids of yeast invertase were replaced by the C-terminal 110 amino acids of gp80. When expressed in D. discoideum, this protein (Inv-gp80) was not GPI-anchored and was retained in a pre-Golgi compartment. Inv-gp80 did, however, display characteristics of a transmembrane protein, suggesting a novel mechanism for its retention. We also expressed a truncated version of the hybrid protein in which the C-terminal 22 amino acids of the Inv-gp80 were deleted. The truncated protein (Inv-gp80stop) was O-glycosylated and secreted. These observations indicate that the hybrid protein is not abnormally folded and demonstrate the importance of the C-terminal 22 amino acids in the retention of Inv-gp80. Together, the data suggest that oligomerization of the protein blocks its GPI anchoring.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford D. A., Alafi C. D., Gamble V. M., Mackay D. J., Rademacher T. W., Williams P. J., Dwek R. A., Barclay A. N., Davis S. J., Somoza C. Site-specific glycosylation of recombinant rat and human soluble CD4 variants expressed in Chinese hamster ovary cells. J Biol Chem. 1993 Feb 15;268(5):3260–3267. [PubMed] [Google Scholar]
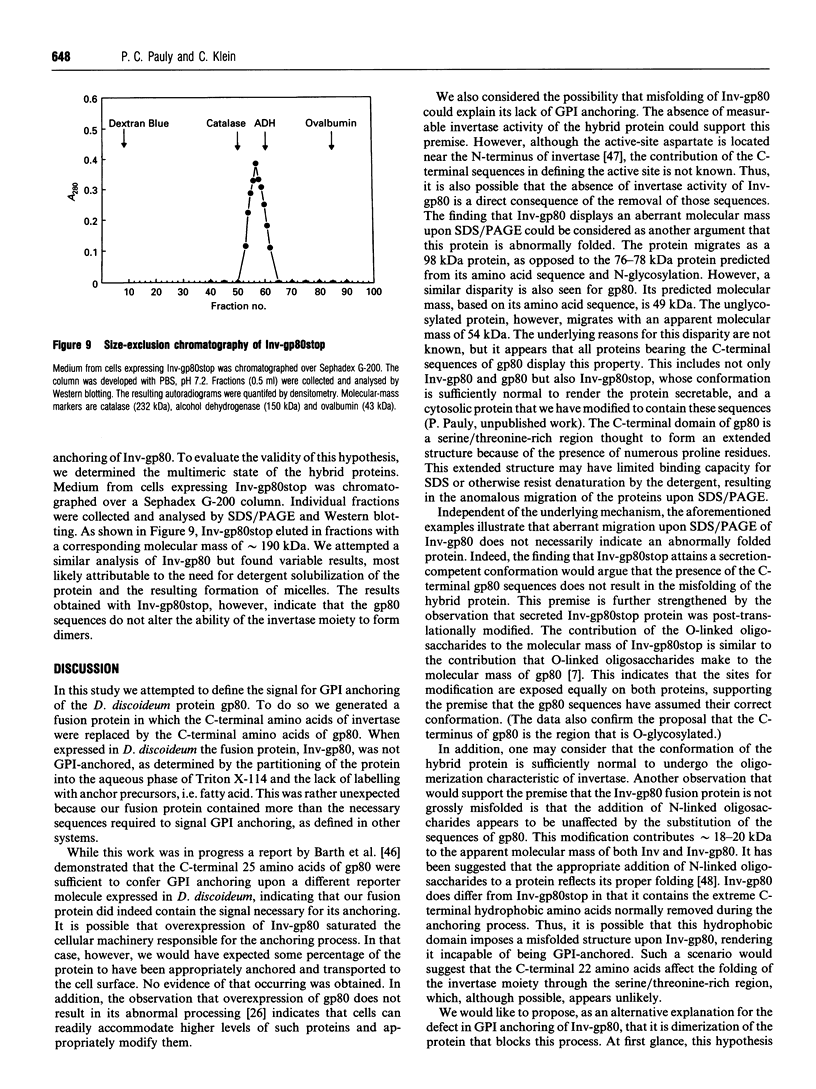
- Barth A., Müller-Taubenberger A., Taranto P., Gerisch G. Replacement of the phospholipid-anchor in the contact site A glycoprotein of D. discoideum by a transmembrane region does not impede cell adhesion but reduces residence time on the cell surface. J Cell Biol. 1994 Jan;124(1-2):205–215. doi: 10.1083/jcb.124.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh M. L., Cepko C. L., Wolf D., Robbins P. W. Expression of the Saccharomyces cerevisiae glycoprotein invertase in mouse fibroblasts: glycosylation, secretion, and enzymatic activity. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3570–3574. doi: 10.1073/pnas.84.11.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. T. Aggregation and differentiation in the cellular slime molds. Annu Rev Microbiol. 1971;25:75–92. doi: 10.1146/annurev.mi.25.100171.000451. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Browne L. H., Sadeghi H., Blumberg D., Williams K. L., Klein C. Re-expression of 117 antigen, a cell surface glycoprotein of aggregating cells, during terminal differentiation of Dictyostelium discoideum prespore cells. Development. 1989 Mar;105(3):657–664. doi: 10.1242/dev.105.3.657. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Davitz M. A., Nussenzweig V., Martin D. W., Jr Signal for attachment of a phospholipid membrane anchor in decay accelerating factor. Science. 1987 Nov 27;238(4831):1280–1283. doi: 10.1126/science.2446389. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Williams S. R. Analysis of the signal for attachment of a glycophospholipid membrane anchor. J Cell Biol. 1989 Apr;108(4):1387–1396. doi: 10.1083/jcb.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. K., Watorek W., Maley F. Factors affecting the oligomeric structure of yeast external invertase. Arch Biochem Biophys. 1983 Jun;223(2):543–555. doi: 10.1016/0003-9861(83)90619-7. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Puoti A., Lester R. L., Desponds C. Two different types of lipid moieties are present in glycophosphoinositol-anchored membrane proteins of Saccharomyces cerevisiae. EMBO J. 1992 Feb;11(2):457–466. doi: 10.1002/j.1460-2075.1992.tb05075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Bron C., Hyman R. No glycolipid anchors are added to Thy-1 glycoprotein in Thy-1-negative mutant thymoma cells of four different complementation classes. Mol Cell Biol. 1988 Feb;8(2):674–678. doi: 10.1128/mcb.8.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Crise B., Ruusala A., Zagouras P., Shaw A., Rose J. K. Oligomerization of glycolipid-anchored and soluble forms of the vesicular stomatitis virus glycoprotein. J Virol. 1989 Dec;63(12):5328–5333. doi: 10.1128/jvi.63.12.5328-5333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Strous G. J. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent, and precedes initial O-glycosylation. J Biol Chem. 1990 Oct 25;265(30):18116–18122. [PubMed] [Google Scholar]
- Delahunty M. D., Stafford F. J., Yuan L. C., Shaz D., Bonifacino J. S. Uncleaved signals for glycosylphosphatidylinositol anchoring cause retention of precursor proteins in the endoplasmic reticulum. J Biol Chem. 1993 Jun 5;268(16):12017–12027. [PubMed] [Google Scholar]
- Esmon P. C., Esmon B. E., Schauer I. E., Taylor A., Schekman R. Structure, assembly, and secretion of octameric invertase. J Biol Chem. 1987 Mar 25;262(9):4387–4394. [PubMed] [Google Scholar]
- Ferguson M. A., Duszenko M., Lamont G. S., Overath P., Cross G. A. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J Biol Chem. 1986 Jan 5;261(1):356–362. [PubMed] [Google Scholar]
- Field M. C., Moran P., Li W., Keller G. A., Caras I. W. Retention and degradation of proteins containing an uncleaved glycosylphosphatidylinositol signal. J Biol Chem. 1994 Apr 8;269(14):10830–10837. [PubMed] [Google Scholar]
- Gerber L. D., Kodukula K., Udenfriend S. Phosphatidylinositol glycan (PI-G) anchored membrane proteins. Amino acid requirements adjacent to the site of cleavage and PI-G attachment in the COOH-terminal signal peptide. J Biol Chem. 1992 Jun 15;267(17):12168–12173. [PubMed] [Google Scholar]
- Gerisch G. Univalent antibody fragments as tools for the analysis of cell interactions in Dictyostelium. Curr Top Dev Biol. 1980;14(Pt 2):243–270. doi: 10.1016/s0070-2153(08)60197-0. [DOI] [PubMed] [Google Scholar]
- Haynes P. A., Gooley A. A., Ferguson M. A., Redmond J. W., Williams K. L. Post-translational modifications of the Dictyostelium discoideum glycoprotein PsA. Glycosylphosphatidylinositol membrane anchor and composition of O-linked oligosaccharides. Eur J Biochem. 1993 Sep 15;216(3):729–737. doi: 10.1111/j.1432-1033.1993.tb18192.x. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Bozzaro S., Merkl R., Wallraff E., Yoshida M., Weinhart U., Gerisch G. Post-translational glycosylation of the contact site A protein of Dictyostelium discoideum is important for stability but not for its function in cell adhesion. EMBO J. 1987 Dec 1;6(12):3663–3671. doi: 10.1002/j.1460-2075.1987.tb02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann H. P., Bozzaro S., Yoshida M., Merkl R., Gerisch G. Two-step glycosylation of the contact site A protein of Dictyostelium discoideum and transport of an incompletely glycosylated form to the cell surface. J Biol Chem. 1987 Dec 5;262(34):16618–16624. [PubMed] [Google Scholar]
- Kemble G. W., Henis Y. I., White J. M. GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J Cell Biol. 1993 Sep;122(6):1253–1265. doi: 10.1083/jcb.122.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lacoste C. H., Graham T., Kaplan A. A sequence in beta-hexosaminidase from Dictyostelium discoideum required for sorting of proteins to a compartment involved in developmentally induced secretion. J Biol Chem. 1992 Mar 25;267(9):5942–5948. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. N., Shaw A. C., Weaver Y. K., Leder P., Abbas A. K. Cytoplasmic tail deletion converts membrane immunoglobulin to a phosphatidylinositol-linked form lacking signaling and efficient antigen internalization functions. J Biol Chem. 1991 May 15;266(14):8856–8860. [PubMed] [Google Scholar]
- Moran P., Caras I. W. A nonfunctional sequence converted to a signal for glycophosphatidylinositol membrane anchor attachment. J Cell Biol. 1991 Oct;115(2):329–336. doi: 10.1083/jcb.115.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Caras I. W. Proteins containing an uncleaved signal for glycophosphatidylinositol membrane anchor attachment are retained in a post-ER compartment. J Cell Biol. 1992 Nov;119(4):763–772. doi: 10.1083/jcb.119.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Caras I. W. Requirements for glycosylphosphatidylinositol attachment are similar but not identical in mammalian cells and parasitic protozoa. J Cell Biol. 1994 Apr;125(2):333–343. doi: 10.1083/jcb.125.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Raab H., Kohr W. J., Caras I. W. Glycophospholipid membrane anchor attachment. Molecular analysis of the cleavage/attachment site. J Biol Chem. 1991 Jan 15;266(2):1250–1257. [PubMed] [Google Scholar]
- Murray B. A., Wheeler S., Jongens T., Loomis W. F. Mutations affecting a surface glycoprotein, gp80, of Dictyostelium discoideum. Mol Cell Biol. 1984 Mar;4(3):514–519. doi: 10.1128/mcb.4.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Nellen W., Datta S., Reymond C., Sivertsen A., Mann S., Crowley T., Firtel R. A. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 1987;28:67–100. doi: 10.1016/s0091-679x(08)61637-4. [DOI] [PubMed] [Google Scholar]
- Noegel A., Gerisch G., Stadler J., Westphal M. Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 1986 Jul;5(7):1473–1476. doi: 10.1002/j.1460-2075.1986.tb04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C., Horvath A., Riezman H. Analysis of the sequence requirements for glycosylphosphatidylinositol anchoring of Saccharomyces cerevisiae Gas1 protein. J Biol Chem. 1993 May 15;268(14):10558–10563. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reddy V. A., Maley F. Identification of an active-site residue in yeast invertase by affinity labeling and site-directed mutagenesis. J Biol Chem. 1990 Jul 5;265(19):10817–10820. [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Roitsch T., Lehle L. Expression of yeast invertase in oocytes from Xenopus laevis. Secretion of active enzyme differing in glycosylation. Eur J Biochem. 1989 May 15;181(3):733–739. doi: 10.1111/j.1432-1033.1989.tb14785.x. [DOI] [PubMed] [Google Scholar]
- Sadeghi H., Klein C. Inhibition of N-linked glycosylation in Dictyostelium discoideum: effects of aggregate formation. Differentiation. 1988 Jul;38(2):99–103. doi: 10.1111/j.1432-0436.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Sadeghi H., da Silva A. M., Klein C. Evidence that a glycolipid tail anchors antigen 117 to the plasma membrane of Dictyostelium discoideum cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5512–5515. doi: 10.1073/pnas.85.15.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Singleton D., Tartakoff A. M. Anchoring and degradation of glycolipid-anchored membrane proteins by L929 versus by LM-TK- mouse fibroblasts: implications for anchor biosynthesis. Mol Cell Biol. 1991 May;11(5):2362–2374. doi: 10.1128/mcb.11.5.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Keenan T. W., Bauer G., Gerisch G. The contact site A glycoprotein of Dictyostelium discoideum carries a phospholipid anchor of a novel type. EMBO J. 1989 Feb;8(2):371–377. doi: 10.1002/j.1460-2075.1989.tb03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R., Carlson M. Nucleotide sequence of the yeast SUC2 gene for invertase. Nucleic Acids Res. 1983 Mar 25;11(6):1943–1954. doi: 10.1093/nar/11.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Subunit structure of external invertase from Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 25;252(12):4409–4412. [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienands J., Hombach J., Radbruch A., Riesterer C., Reth M. Molecular components of the B cell antigen receptor complex of class IgD differ partly from those of IgM. EMBO J. 1990 Feb;9(2):449–455. doi: 10.1002/j.1460-2075.1990.tb08130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienands J., Reth M. Glycosyl-phosphatidylinositol linkage as a mechanism for cell-surface expression of immunoglobulin D. Nature. 1992 Mar 19;356(6366):246–248. doi: 10.1038/356246a0. [DOI] [PubMed] [Google Scholar]
- da Silva A. M., Klein C. Cell adhesion in transformed D. discoideum cells: expression of gp80 and its biochemical characterization. Dev Biol. 1990 Jul;140(1):139–148. doi: 10.1016/0012-1606(90)90061-m. [DOI] [PubMed] [Google Scholar]
- da Silva A. M., Klein C. Characterization of a glycosyl-phosphatidylinositol degrading activity in Dictyostelium discoideum membranes. Exp Cell Res. 1989 Dec;185(2):464–472. doi: 10.1016/0014-4827(89)90315-7. [DOI] [PubMed] [Google Scholar]
- de Lederkremer R. M., Lima C., Ramirez M. I., Ferguson M. A., Homans S. W., Thomas-Oates J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi Epimastigotes. J Biol Chem. 1991 Dec 15;266(35):23670–23675. [PubMed] [Google Scholar]