Abstract
Enzyme activities involved in quantitative and qualitative flux of sugars into cell wall polysaccharides were determined following elicitor treatment of suspension cultured cells of French bean (Phaseolus vulgaris L.). Two subsets of activities were examined: the first were involved in synthesis and metabolism of UDP-glucose and the provision of the pool of UDP-sugars, and the second a selection of membrane-bound glycosyltransferases involved in the synthesis of pectins, hemicelluloses and glucans of the primary cell wall. Of the first group, only UDP-glucose dehydrogenase (EC 1.1.1.22) showed any significant induction in response to elicitor treatment, sucrose synthase (EC 2.4.1.13), UDP-glucuronate decarboxylase (EC 4.1.1.35), UDP-glucose and UDP-xylose 4-epimerases (EC 5.1.3.2 and EC 5.1.3.5 respectively) did not change in activity significantly over the time course. In contrast, enzymes of the second group showed a more complex response. Callose synthase (glucan synthase II, EC 2.4.1.12) increased in activity, as has been shown in other systems, while arabinan synthase (EC 2.4.1.-), xylan synthase (EC 2.4.1.72), xyloglucan synthase (EC 2.4.1.72) and glucan synthase I (EC 2.4.1.12) activities were rapidly depleted from membranes within 3 h following elicitor action. This rapid turnover of activity was striking, indicating that the half-life of such enzymes can be short and that elicitor action causes substantial perturbation of some membrane activities. Glucan synthase I activity appears to increase in the later stages over the time period measured, indicating some recovery of this metabolism.
Full text
PDF

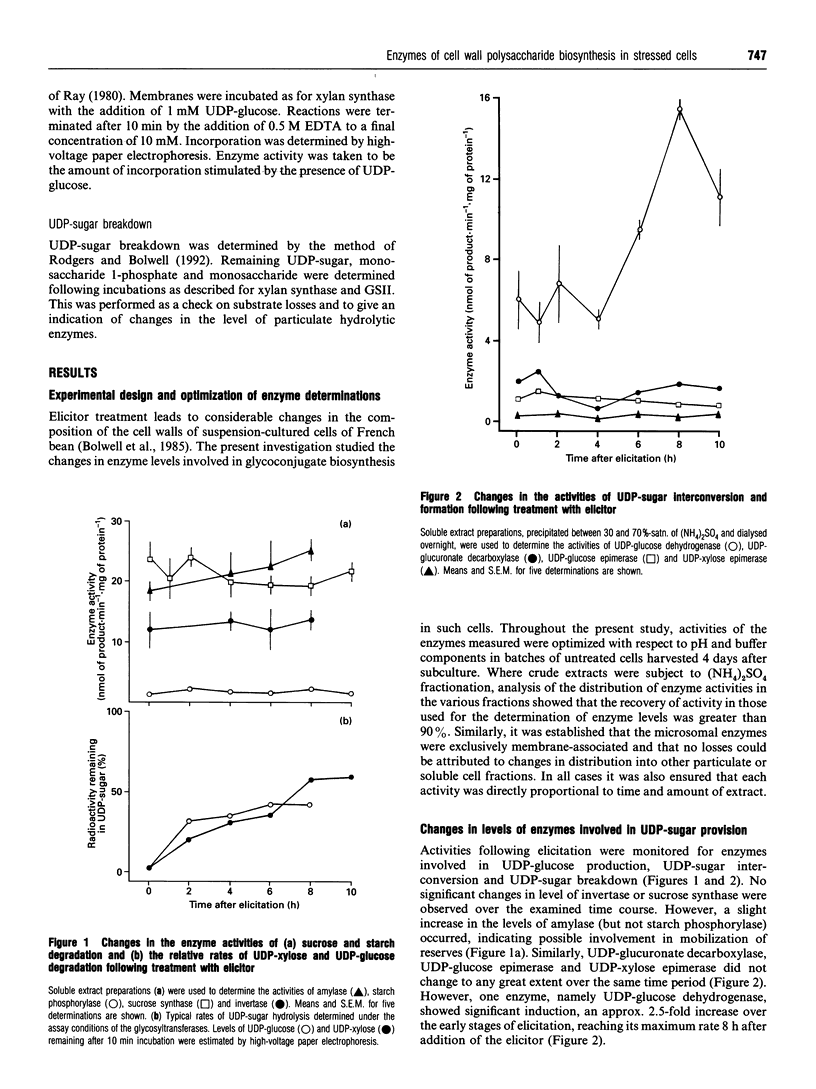
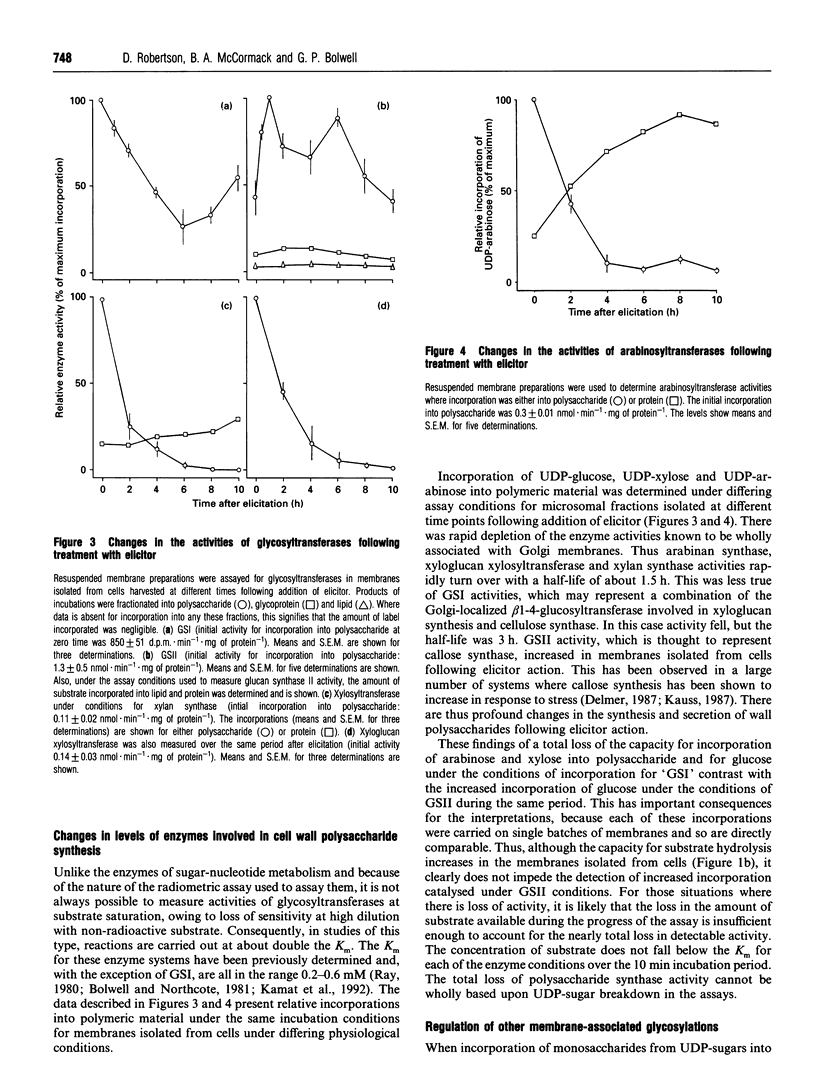


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolwell G. P., Northcote D. H. Arabinan synthase and xylan synthase activities of Phaseolus vulgaris. Subcellular localization and possible mechanism of action. Biochem J. 1983 Feb 15;210(2):497–507. doi: 10.1042/bj2100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Induction by growth factors of polysaccharide synthases in bean cell suspension cultures. Biochem J. 1983 Feb 15;210(2):509–515. doi: 10.1042/bj2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Robbins M. P., Dixon R. A. Metabolic changes in elicitor-treated bean cells. Enzymic responses associated with rapid changes in cell wall components. Eur J Biochem. 1985 May 2;148(3):571–578. doi: 10.1111/j.1432-1033.1985.tb08878.x. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kjellbom P., Lamb C. J. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992 Jul 10;70(1):21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Carpita N. C., Delmer D. P. Concentration and metabolic turnover of UDP-glucose in developing cotton fibers. J Biol Chem. 1981 Jan 10;256(1):308–315. [PubMed] [Google Scholar]
- Dalessandro G., Northcote D. H. Changes in enzymic activities of nucleoside diphosphate sugar interconversions during differentiation of cambium to xylem in sycamore and poplar. Biochem J. 1977 Feb 15;162(2):267–279. doi: 10.1042/bj1620267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat U., Garg R., Sharma C. B. Purification to homogeneity and characterization of a 1,3-beta-glucan (callose) synthase from germinating Arachis hypogaea cotyledons. Arch Biochem Biophys. 1992 Nov 1;298(2):731–739. doi: 10.1016/0003-9861(92)90473-a. [DOI] [PubMed] [Google Scholar]
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Cooperative action of beta-glucan synthetase and UDP-xylose xylosyl transferase of Golgi membranes in the synthesis of xyloglucan-like polysaccharide. Biochim Biophys Acta. 1980 May 22;629(3):431–444. doi: 10.1016/0304-4165(80)90149-x. [DOI] [PubMed] [Google Scholar]
- Robinson N. L., Hewitt J. D., Bennett A. B. Sink metabolism in tomato fruit : I. Developmental changes in carbohydrate metabolizing enzymes. Plant Physiol. 1988 Jul;87(3):727–730. doi: 10.1104/pp.87.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers M. W., Bolwell G. P. Partial purification of Golgi-bound arabinosyltransferase and two isoforms of xylosyltransferase from French bean (Phaseolus vulgaris L.). Biochem J. 1992 Dec 15;288(Pt 3):817–822. doi: 10.1042/bj2880817. [DOI] [PMC free article] [PubMed] [Google Scholar]


