Abstract
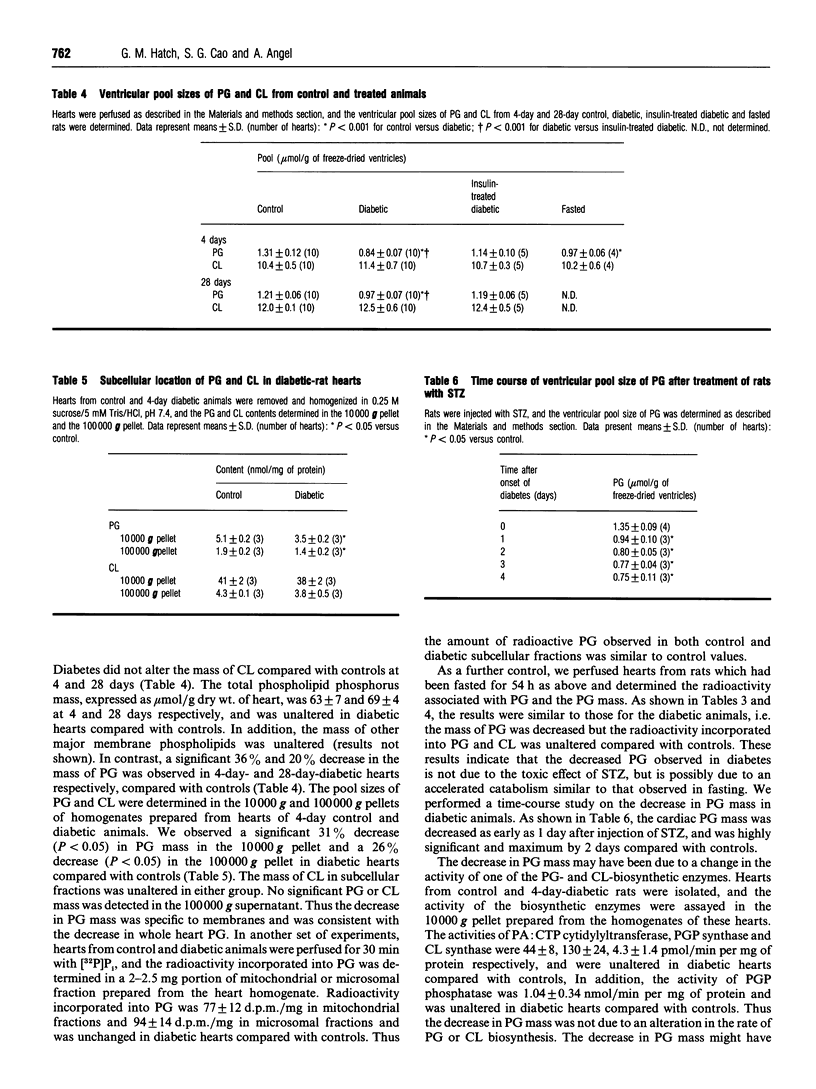
Biosynthesis of phosphatidylglycerol (PG) and cardiolipin (CL) were investigated in perfused hearts of diabetic rats 4 days or 28 days after streptozotocin injection. Sham-injected and insulin-treated diabetic rats were used as controls. In addition, another group of rats fasted for 54 h was examined. Isolated rat hearts from these groups were perfused for 30 min with [32P]P(i), and the radioactivity incorporated into PG and CL and their pool sizes were determined in heart ventricles. There was no difference in the amount of radioactivity incorporated into CL, PG or other phospholipids between all groups. In addition, the pool sizes of CL and other phospholipids were unaltered. However, a striking decrease in the pool size of PG was observed in both diabetic and fasted rats compared to sham- and insulin-treated controls at 4 days after streptozotocin injection. The decrease in PG mass in diabetic rats was rapid (within 24-48 h) and was localized to cardiac membranes. Diabetes did not affect the activity of the enzymes of PG and CL biosynthesis in the mitochondrial fraction, or phospholipase A activity in subcellular fractions prepared from rat heart homogenates. In addition, pulse-chase experiments confirmed that diabetes did not affect the rate of new PG or CL biosynthesis. Since radioactivity associated with PG was unaltered in continuous-pulse perfusion experiments, a calculated 1.8-fold increase in the specific radioactivity of cardiac PG was observed in the hearts of acute diabetic rats compared with controls. Since the radioactivity incorporated into PG and CL, and the rate of CL biosynthesis, were unaltered in diabetic-rat hearts compared with controls, new CL was probably synthesized from newly synthesized PG. We postulate the existence of distinct pools of PG in the heart, and that the pool of newly synthesized PG used for CL biosynthesis does not appear to mix immediately with the pre-existing pool of PG in the isolated intact rat heart.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison T. B., Bruttig S. P., Crass M. F., 3rd, Eliot R. S., Shipp J. C. Reduced high-energy phosphate levels in rat hearts. I. Effects of alloxan diabetes. Am J Physiol. 1976 Jun;230(6):1744–1750. doi: 10.1152/ajplegacy.1976.230.6.1744. [DOI] [PubMed] [Google Scholar]
- Batenburg J. J., Klazinga W., van Golde L. M. Regulation and location of phosphatidylglycerol and phosphatidylinositol synthesis in type II cells isolated from fetal rat lung. Biochim Biophys Acta. 1985 Jan 9;833(1):17–24. doi: 10.1016/0005-2760(85)90248-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Belunis C. J. Phosphatidylglycerophosphate synthase activity in Saccharomyces cerevisiae. Can J Microbiol. 1983 Oct;29(10):1452–1457. doi: 10.1139/m83-222. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Kelley M. J. CDPdiacylglycerol synthase from yeast. Methods Enzymol. 1992;209:242–247. doi: 10.1016/0076-6879(92)09030-7. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Pierce G. N., Dhalla K. S., Dhalla N. S. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983 Jun;244(6):E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullestad L., Kjekshus J. Myokardaffeksjon ved diabetes mellitus. Tidsskr Nor Laegeforen. 1992 Mar 20;112(8):1016–1019. [PubMed] [Google Scholar]
- Hatch G. M. Cardiolipin biosynthesis in the isolated heart. Biochem J. 1994 Jan 1;297(Pt 1):201–208. doi: 10.1042/bj2970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. M., Choy P. C. Phosphocholine phosphatase and alkaline phosphatase are different enzymes in hamster heart. Lipids. 1987 Sep;22(9):672–676. doi: 10.1007/BF02533949. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Van den Bosch H., Van Deenen L. L. Biosynthesis of cardiolipin in liver mitochondria. Biochim Biophys Acta. 1971 Jun 8;239(1):113–119. doi: 10.1016/0005-2760(71)90201-3. [DOI] [PubMed] [Google Scholar]
- KIYASU J. Y., PIERINGER R. A., PAULUS H., KENNEDY E. P. The biosynthesis of phosphatidylglycerol. J Biol Chem. 1963 Jul;238:2293–2298. [PubMed] [Google Scholar]
- Kim D. K., Bonventre J. V. Purification of a 100 kDa phospholipase A2 from spleen, lung and kidney: antiserum raised to pig spleen phospholipase A2 recognizes a similar form in bovine lung, kidney and platelets, and immunoprecipitates phospholipase A2 activity. Biochem J. 1993 Aug 15;294(Pt 1):261–270. doi: 10.1042/bj2940261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekka M., Tokumura A., Tsuji H., Hanahan D. J. Isolation of a phospholipid inhibitor of platelet activating factor-induced activity from perfused rat liver: identification as phosphatidylglycerol. Arch Biochem Biophys. 1993 May;302(2):380–384. doi: 10.1006/abbi.1993.1227. [DOI] [PubMed] [Google Scholar]
- Makino N., Dhalla K. S., Elimban V., Dhalla N. S. Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol. 1987 Aug;253(2 Pt 1):E202–E207. doi: 10.1152/ajpendo.1987.253.2.E202. [DOI] [PubMed] [Google Scholar]
- Melki S. A., Abumrad N. A. Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J Lipid Res. 1993 Sep;34(9):1527–1534. [PubMed] [Google Scholar]
- Müller M., Moser R., Cheneval D., Carafoli E. Cardiolipin is the membrane receptor for mitochondrial creatine phosphokinase. J Biol Chem. 1985 Mar 25;260(6):3839–3843. [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T., Nishijima M., Suzuki K., Akamatsu Y. Mitochondrial dysfunction of a cultured Chinese hamster ovary cell mutant deficient in cardiolipin. J Biol Chem. 1993 Oct 25;268(30):22914–22919. [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Schlame M., Hostetler K. Y. Mammalian cardiolipin biosynthesis. Methods Enzymol. 1992;209:330–337. doi: 10.1016/0076-6879(92)09041-z. [DOI] [PubMed] [Google Scholar]
- Trotz M., Hein L., Hostetler K. Y. Solubilization and partial characterization of phospholipase A from rat heart sarcoplasmic reticulum. Biochim Biophys Acta. 1988 Sep 23;962(2):248–257. doi: 10.1016/0005-2760(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Vettor R., Martini C., Calò L., Cantaro S., Macor C., De Palo C., Sicolo N., Scandellari C., Federspil G. Increased muscular phospholipase A2 activity in diabetic rats. Diabete Metab. 1992 May-Jun;18(3):213–217. [PubMed] [Google Scholar]
- Vik S. B., Georgevich G., Capaldi R. A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J. 1993 Aug 15;294(Pt 1):1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Hilkmann H. Rapid attenuation of receptor-induced diacylglycerol and phosphatidic acid by phospholipase D-mediated transphosphatidylation: formation of bisphosphatidic acid. EMBO J. 1993 Jul;12(7):2655–2662. doi: 10.1002/j.1460-2075.1993.tb05926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


