Abstract
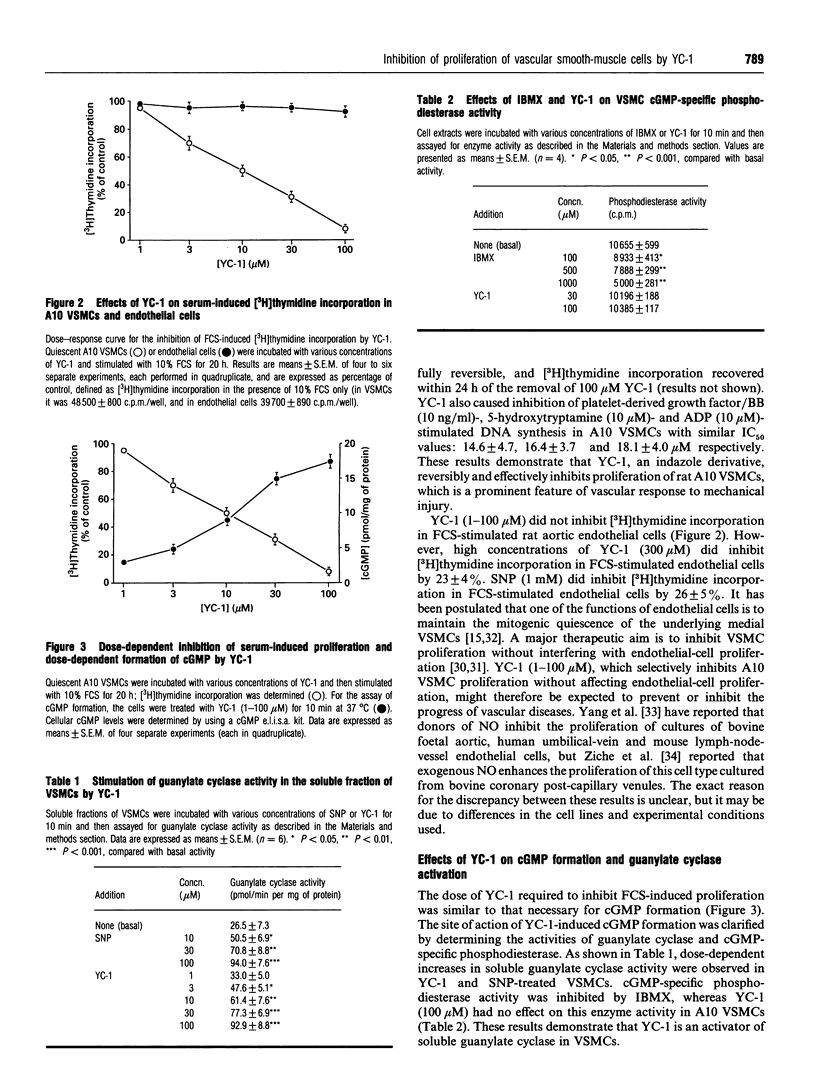
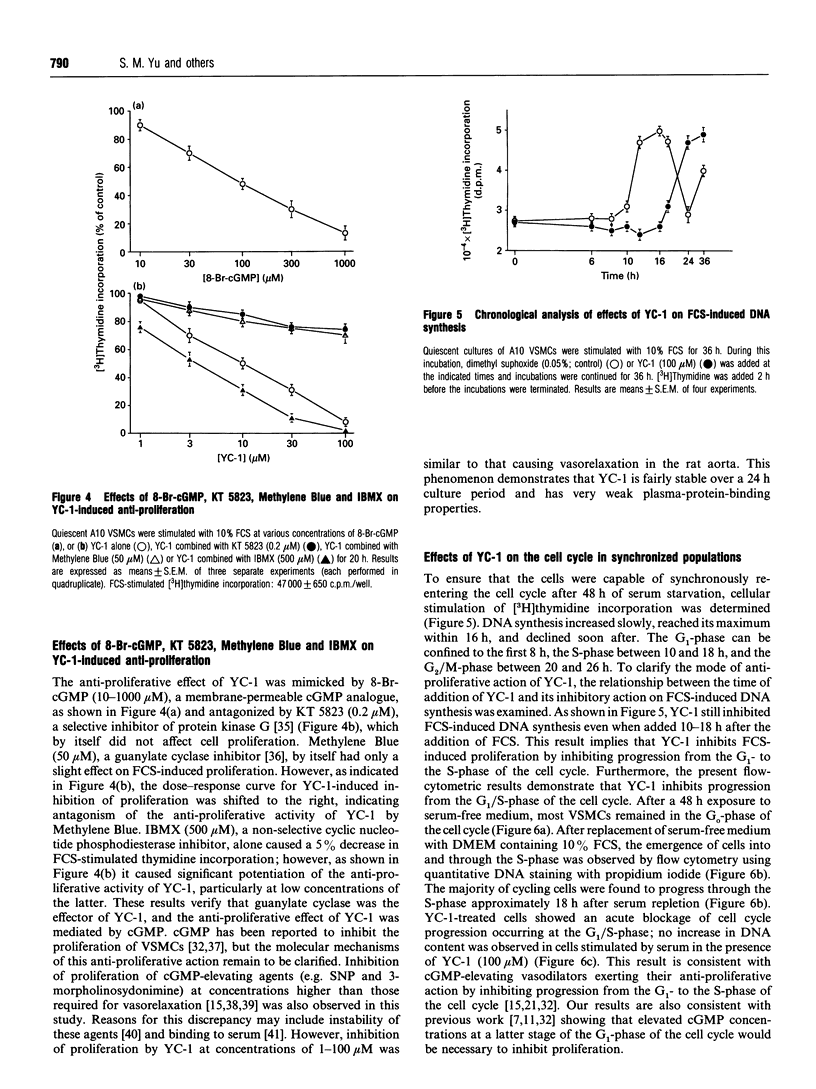
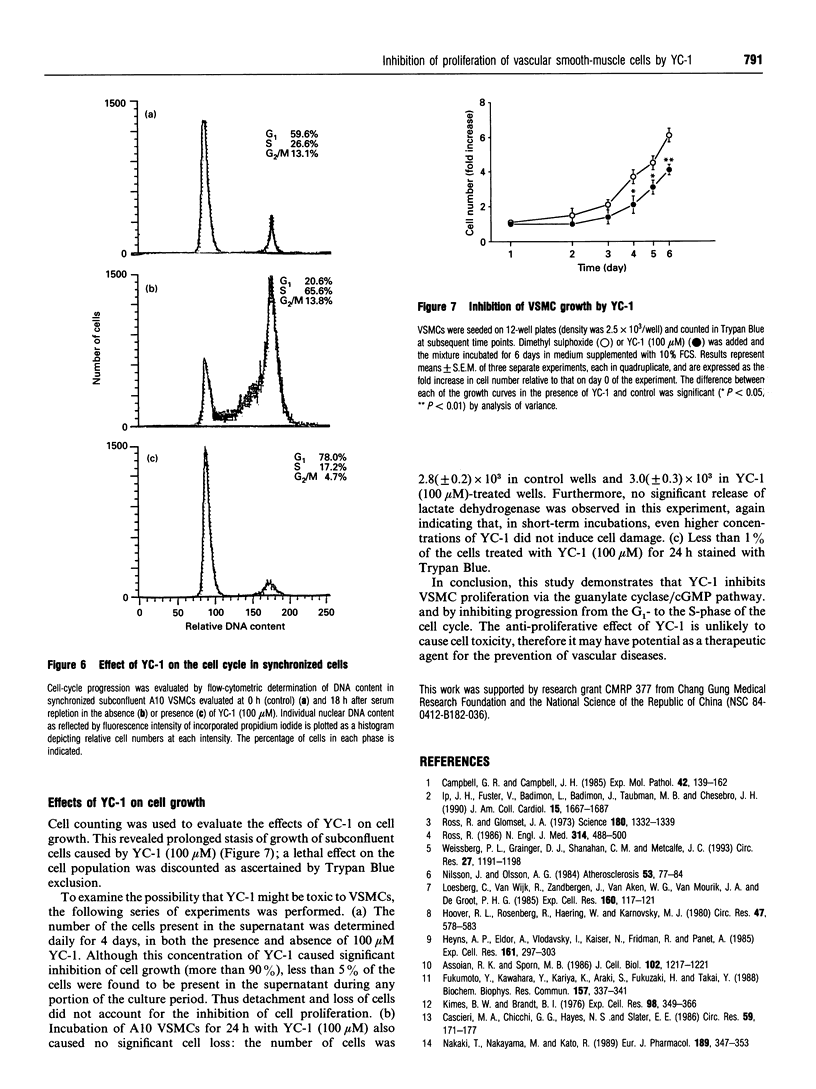
An indazole derivative, YC-1, was identified in this study to be capable of reversibly and effectively inhibiting proliferation of rat A10 vascular smooth-muscle cells (VSMCs) in vitro. YC-1 (1-100 microM) dose-dependently inhibited [3H]thymidine incorporation into DNA in rat A10 VSMCs that were synchronized by serum depletion and then restimulated by addition of 10% foetal calf serum (FCS), whereas FCS-induced [3H]thymidine incorporation into rat synchronized endothelial cells was unaffected by this agent. The dose of YC-1 required to cause inhibition of FCS-induced proliferation was similar to that necessary for the formation of cellular cyclic GMP (cGMP). Guanylate cyclase activity in soluble fractions of VSMCs was activated by YC-1 (1-100 microM), whereas cGMP-specific phosphodiesterase activity was unaffected by this compound. The anti-proliferative effect of YC-1 was mimicked by 8-bromo-cGMP, a membrane-permeable cGMP analogue, and was antagonized by KT 5823 (0.2 microM), a selective inhibitor of protein kinase G. The anti-proliferative effect of YC-1 was also antagonized by Methylene Blue (50 microM), a guanylate cyclase inhibitor, and was potentiated by 3-isobutyl-1-methylxanthine (500 microM), a phosphodiesterase inhibitor. These results verified that YC-1 is a direct soluble guanylate cyclase activator in A10 VSMCs, and the anti-proliferative effect of YC-1 is mediated by cGMP. YC-1 still inhibited FCS-induced DNA synthesis even when added 10-18 h after restimulation of the serum-deprived A10 VSMCs with 10% FCS. Flow cytometry in synchronized populations revealed an acute blockage of FCS-inducible cell-cycle progression at a point in the G1/S-phase in YC-1 (100 microM)-treated cells. The inhibition of proliferation by YC-1 was demonstrated to be independent of cell damage, as documented by several criteria of cell viability. In conclusion, YC-1 reversibly and effectively inhibited the proliferation of VSMCs, suggesting that it has potential as a therapeutic agent in the prevention of vascular diseases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assender J. W., Southgate K. M., Hallett M. B., Newby A. C. Inhibition of proliferation, but not of Ca2+ mobilization, by cyclic AMP and GMP in rabbit aortic smooth-muscle cells. Biochem J. 1992 Dec 1;288(Pt 2):527–532. doi: 10.1042/bj2880527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol. 1985 Apr;42(2):139–162. doi: 10.1016/0014-4800(85)90023-1. [DOI] [PubMed] [Google Scholar]
- Cascieri M. A., Chicchi G. G., Hayes N. S., Slater E. E. Stimulation of DNA synthesis in rat A10 vascular smooth muscle cells by threonine-59 insulin-like growth factor I. Circ Res. 1986 Aug;59(2):171–177. doi: 10.1161/01.res.59.2.171. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Edwards D. H., Griffith T. M., Ryley H. C., Henderson A. H. Haptoglobin-haemoglobin complex in human plasma inhibits endothelium dependent relaxation: evidence that endothelium derived relaxing factor acts as a local autocoid. Cardiovasc Res. 1986 Aug;20(8):549–556. doi: 10.1093/cvr/20.8.549. [DOI] [PubMed] [Google Scholar]
- Friedman D. L. Role of cyclic nucleotides in cell growth and differentiation. Physiol Rev. 1976 Oct;56(4):652–708. doi: 10.1152/physrev.1976.56.4.652. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kawahara Y., Kariya K., Araki S., Fukuzaki H., Takai Y. Independent inhibition of DNA synthesis by protein kinase C, cyclic AMP and interferon alpha/beta in rabbit aortic smooth muscle cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):337–345. doi: 10.1016/s0006-291x(88)80052-4. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzer R., Hamet P., Ross A. H., Lawson J. A., Hardman J. G. Calcium-induced release from platelet membranes of fatty acids that modulate soluble guanylate cyclase. J Pharmacol Exp Ther. 1983 Jul;226(1):180–186. [PubMed] [Google Scholar]
- Grainger D. J., Hesketh T. R., Metcalfe J. C., Weissberg P. L. A large accumulation of non-muscle myosin occurs at first entry into M phase in rat vascular smooth-muscle cells. Biochem J. 1991 Jul 1;277(Pt 1):145–151. doi: 10.1042/bj2770145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger D. J., Hesketh T. R., Weissberg P. L., Metcalfe J. C. Hexamethylenebisacetamide selectively inhibits the proliferation of human and rat vascular smooth-muscle cells. Biochem J. 1992 Apr 15;283(Pt 2):403–408. doi: 10.1042/bj2830403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyns A. D., Eldor A., Vlodavsky I., Kaiser N., Fridman R., Panet A. The antiproliferative effect of interferon and the mitogenic activity of growth factors are independent cell cycle events. Studies with vascular smooth muscle cells and endothelial cells. Exp Cell Res. 1985 Dec;161(2):297–306. doi: 10.1016/0014-4827(85)90087-4. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Badimon L., Badimon J., Taubman M. B., Chesebro J. H. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990 Jun;15(7):1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- Jonzon B., Nilsson J., Fredholm B. B. Adenosine receptor-mediated changes in cyclic AMP production and DNA synthesis in cultured arterial smooth muscle cells. J Cell Physiol. 1985 Sep;124(3):451–456. doi: 10.1002/jcp.1041240314. [DOI] [PubMed] [Google Scholar]
- Kariya K., Kawahara Y., Araki S., Fukuzaki H., Takai Y. Antiproliferative action of cyclic GMP-elevating vasodilators in cultured rabbit aortic smooth muscle cells. Atherosclerosis. 1989 Dec;80(2):143–147. doi: 10.1016/0021-9150(89)90022-1. [DOI] [PubMed] [Google Scholar]
- Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987 Jan 30;142(2):436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Brandt B. L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res. 1976 Mar 15;98(2):349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- Ko F. N., Wu C. C., Kuo S. C., Lee F. Y., Teng C. M. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994 Dec 15;84(12):4226–4233. [PubMed] [Google Scholar]
- Kondo T., Konishi F., Inui H., Inagami T. Differing signal transductions elicited by three isoforms of platelet-derived growth factor in vascular smooth muscle cells. J Biol Chem. 1993 Feb 25;268(6):4458–4464. [PubMed] [Google Scholar]
- Loesberg C., van Wijk R., Zandbergen J., van Aken W. G., van Mourik J. A., de Groot P. G. Cell cycle-dependent inhibition of human vascular smooth muscle cell proliferation by prostaglandin E1. Exp Cell Res. 1985 Sep;160(1):117–125. doi: 10.1016/0014-4827(85)90241-1. [DOI] [PubMed] [Google Scholar]
- March K. L., Wilensky R. L., Roeske R. W., Hathaway D. R. Effects of thiol protease inhibitors on cell cycle and proliferation of vascular smooth muscle cells in culture. Circ Res. 1993 Feb;72(2):413–423. doi: 10.1161/01.res.72.2.413. [DOI] [PubMed] [Google Scholar]
- Moore J. B., Jr, Fuller B. L., Falotico R., Tolman E. L. Inhibition of rabbit platelet phosphodiesterase activity and aggregation by calcium channel blockers. Thromb Res. 1985 Nov 1;40(3):401–411. doi: 10.1016/0049-3848(85)90275-0. [DOI] [PubMed] [Google Scholar]
- Morinelli T. A., Zhang L. M., Newman W. H., Meier K. E. Thromboxane A2/prostaglandin H2-stimulated mitogenesis of coronary artery smooth muscle cells involves activation of mitogen-activated protein kinase and S6 kinase. J Biol Chem. 1994 Feb 25;269(8):5693–5698. [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990 Dec 15;189(6):347–353. doi: 10.1016/0922-4106(90)90031-r. [DOI] [PubMed] [Google Scholar]
- Nemecek G. M., Coughlin S. R., Handley D. A., Moskowitz M. A. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc Natl Acad Sci U S A. 1986 Feb;83(3):674–678. doi: 10.1073/pnas.83.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., Olsson A. G. Prostaglandin E1 inhibits DNA synthesis in arterial smooth muscle cells stimulated with platelet-derived growth factor. Atherosclerosis. 1984 Oct;53(1):77–82. doi: 10.1016/0021-9150(84)90107-2. [DOI] [PubMed] [Google Scholar]
- Noack E., Feelisch M. Molecular aspects underlying the vasodilator action of molsidomine. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S1–S5. [PubMed] [Google Scholar]
- Owen N. E. Effect of prostaglandin E1 on DNA synthesis in vascular smooth muscle cells. Am J Physiol. 1986 Apr;250(4 Pt 1):C584–C588. doi: 10.1152/ajpcell.1986.250.4.C584. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Weissberg P. L., Grainger D. J., Shanahan C. M., Metcalfe J. C. Approaches to the development of selective inhibitors of vascular smooth muscle cell proliferation. Cardiovasc Res. 1993 Jul;27(7):1191–1198. doi: 10.1093/cvr/27.7.1191. [DOI] [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- Yang W., Ando J., Korenaga R., Toyo-oka T., Kamiya A. Exogenous nitric oxide inhibits proliferation of cultured vascular endothelial cells. Biochem Biophys Res Commun. 1994 Sep 15;203(2):1160–1167. doi: 10.1006/bbrc.1994.2304. [DOI] [PubMed] [Google Scholar]
- Yoshina S., Tanaka A., Kuo S. C. [Studies on heterocyclic compounds. XXXIV. Synthesis of furo[3,2-c]pyrazole derivatives. (2). Electrophilic substitution of 1,3-diphenylfuro[3,2-c]pyrazole (author's transl)]. Yakugaku Zasshi. 1978 Feb;98(2):204–209. doi: 10.1248/yakushi1947.98.2_204. [DOI] [PubMed] [Google Scholar]
- Yu S. M., Chen C. C., Huang Y. L., Tsai C. W., Lin C. H., Huang T. F., Teng C. M. Vasorelaxing effect in rat thoracic aorta caused by denudatin B, isolated from the Chinese herb, magnolia fargesii. Eur J Pharmacol. 1990 Oct 2;187(1):39–47. doi: 10.1016/0014-2999(90)90338-7. [DOI] [PubMed] [Google Scholar]
- Ziche M., Morbidelli L., Masini E., Granger H., Geppetti P., Ledda F. Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem Biophys Res Commun. 1993 May 14;192(3):1198–1203. doi: 10.1006/bbrc.1993.1543. [DOI] [PubMed] [Google Scholar]


