Abstract
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and International Deep Endometriosis Analysis (IDEA) group, the European Endometriosis League (EEL), the European Society for Gynaecological Endoscopy (ESGE), the European Society of Human Reproduction and Embryology (ESHRE), the International Society for Gynecologic Endoscopy (ISGE), the American Association of Gynecologic Laparoscopists (AAGL) and the European Society of Urogenital Radiology (ESUR) elected an international, multidisciplinary panel of gynecological surgeons, sonographers and radiologists, including a steering committee, which searched the literature for relevant articles in order to review the literature and provide evidence-based and clinically relevant statements on the use of imaging techniques for non-invasive diagnosis and classification of pelvic deep endometriosis. Preliminary statements were drafted based on a review of the relevant literature. Following two rounds of revisions and voting orchestrated by chairs of the participating societies, consensus statements were finalized. A final version of the document was then resubmitted to the society chairs for approval.
Twenty statements were drafted, of which 14 reached strong and three moderate agreement after the first voting round. The remaining three statements were discussed by all members of the steering committee and society chairs and rephrased, followed by an additional round of voting. At the conclusion of the process, 14 statements had strong and five statements moderate agreement, with one statement left in equipoise. This consensus work aims to guide clinicians involved in treating women with suspected endometriosis during patient assessment, counselling and planning of surgical treatment strategies.
Introduction
Reducing the diagnostic delay of endometriosis to facilitate adequate action requires a shift from a surgically or lesion-oriented diagnosis to a more comprehensive diagnosis, taking into account not only symptoms and signs, but also non-invasive findings on physical examination and imaging. The latter are contributing increasingly to clinical diagnosis and timely intervention (Agarwal et al., 2019). Various non-invasive imaging techniques have been advocated over the past few decades for non-surgical visualization of pelvic endometriosis. Amongst these, ultrasound, primarily using a transvaginal approach, is the imaging modality used most commonly for investigation of women with suspected endometriosis, alongside magnetic resonance imaging (MRI) (Bielen et al., 2020) and, less commonly, computed tomography (CT) (Pascoal et al., 2022) or other radiological techniques, such as barium enema and intravenous urography.
It is of pivotal importance for patient counselling and planning of treatment strategies to achieve an accurate diagnosis of endometriosis on imaging, especially deep endometriosis (DE), which is observed in approximately 20% of cases of endometriosis (Abrao et al., 2015). Prior to surgery, the diagnosis of DE can be used to predict operative difficulty and, equally important, in the context of infertility, particularly involving ovarian endometriosis, it can assist in the decision regarding whether to treat with surgery or apply assisted reproductive technologies, especially when used in combination with predictive tools, such as the Endometriosis Fertility Index (EFI) (Adamson and Pasta, 2010; Tomassetti et al., 2021; Vesali et al., 2020). The study of Goncalves et al. (2021) concluded that systematic evaluation of endometriosis by transvaginal ultrasound (TVS) can accurately replace diagnostic laparoscopy, particularly for DE and ovarian endometriosis. This view is also supported by the recently published updated version of the European Society of Human Reproduction and Embryology (ESHRE) Endometriosis Guideline (Becker et al., 2022), which states that the requirement for histological confirmation for diagnosis of endometriosis is in need of refinement due to ‘…advances in the quality and availability of imaging modalities for at least some forms of endometriosis on the one hand and the operative risk, limited access to highly qualified surgeons and financial implications on the other’.
Ideally, patients with severe DE should be seen at a tertiary referral centre, as they may benefit from input from a multidisciplinary team comprising gynaecologists, urologists, colorectal surgeons and specialists in reproductive medicine and imaging (Bendifallah et al., 2018), hence the importance of detailed presurgical characterisation and classification of endometriosis, especially DE (Abrao et al., 2015). Several attempts have been made to evaluate the use of current classification and scoring systems incorporating non-invasive imaging techniques in order to facilitate these processes (Hudelist et al., 2021a). However, the environmental impact of non-invasive imaging techniques for endometriosis should be recognised in these times of climate crisis. A recent study by McAlister et al. (2022) calculated the carbon footprint of imaging by MRI, CT, and ultrasound in Australia. Of the three modalities, MRI exhibited the largest carbon footprint, followed by CT and then ultrasound. Their impact is attributable mainly to energy consumption and, to some extent, to consumables. Hence, when choosing an imaging technique for patients with suspected endometriosis, physicians should take into consideration that ultrasound has the smallest environmental impact.
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and International Deep Endometriosis Analysis (IDEA) group, the European Society for Gynaecological Endoscopy (ESGE), the European Endometriosis League (EEL), the International Society for Gynecologic Endoscopy (ISGE), ESHRE, the European Society of Urogenital Radiology (ESUR) and the American Association of Gynecologic Laparoscopists (AAGL) therefore formed a working group to develop evidence-based statements to guide the use of non-invasive imaging techniques for diagnosis and classification of pelvic DE, presented in this joint Consensus Statement. Adenomyosis, ovarian endometrioma, superficial and extrapelvic endometriosis, adhesions, biomarkers, economic analysis of these techniques and pathohistological and/or surgical methods for classification and diagnosis of endometriosis are not considered herein.
Responsibilities
The following statements derive from a consensus process that included all listed authors and collaborators and representatives from the respective societies and reflect current evidence-based practice and approaches for the non-invasive diagnosis and classification of endometriosis using imaging techniques. We strongly recommend that clinicians in everyday clinical practice apply independent medical judgement and consider the individual situation and needs of the patient when consulting these statements. All authors listed in this work disclaim any responsibility for its use or application and any clinical decisions deriving from the use of these statements.
Methods
This Consensus Statement was developed in accordance with a protocol used in a previously published Consensus Statement (Timmerman et al., 2021), and involves societies also represented in that work. Using a six-step protocol chaired and organized by Professors George Condous (G.C.) and Gernot Hudelist (G.H.), an international and multidisciplinary working group was established and orchestrated by chairs of each society, referred to herein as society working-group chairs (G. Condous, ISUOG, IDEA; J. Keckstein, E. Saridogan, ESGE; H. Krentel, G. Hudelist, EEL; C. Becker, C. Tomassetti, ESHRE; B.J. van Herendael, ISGE; M.S. Abrao, M. Malzoni, AAGL; I. Thomassin-Naggara, ESUR). The working group included 53 experts with extensive expertise in the field of diagnosis and/or surgical treatment of endometriosis, reflected by research, clinical expertise, administrative responsibilities and society leadership positions, and comprised 10 radiologists with a special interest and expertise in MRI and TVS, 12 gynaecologists with a special interest and expertise in gynaecological ultrasound, 13 gynaecologists with extensive experience in surgery for DE and gynaecological ultrasound and 18 gynaecologists focused exclusively on surgery for DE.
A systematic literature review of relevant studies published from inception to February 2023 was carried out by the coordinating chairs (G.C., G.H.) and the joint first author, Bassem Gerges (B.G.), using the MEDLINE, EMBASE, Google Scholar, PubMed, and Scopus databases (Appendix 1). The protocol and following methodology, being standard for systematic reviews and meta-analyses, have been described in detail in a previously published study (Gerges et al., 2021a). The literature search was limited to publications in the English language. Editorials, letters and case reports were excluded, with priority given to systematic reviews, meta-analyses and validating cohort studies. Additionally, the reference list of each identified article was reviewed for other potentially relevant articles. The coordinating chairs (G.C., G.H.) and joint first author (B.G.) formulated the preliminary consensus statements and were responsible for the first draft of the manuscript. This was followed by distribution of the manuscript to the society chairs, who again distributed and discussed it with all group members, followed by a first round of revisions coordinated by the society chairs. Group members had the opportunity to provide comments and suggestions with their resubmitted versions of the manuscript draft, and statements were modified if there was a lack of consensus among them. The society working-group chairs then submitted the results and comments of the first draft to the coordinating chairs (G.C., G.H.) and joint first author (B.G.) and suggested revisions of the statements if necessary. A revised version of the manuscript was produced and resubmitted to working-group chairs, and thereby all group members, and the process was repeated. Based on the results of the second round, the work and consensus statements were finalised, resulting in 20 statements achieved during this process. Society group members were then able to vote in a binary fashion (agree/disagree), or to abstain from voting in cases of conflict of interest. Statements were classified as having strong agreement (more than 80% of voters agreed), moderate agreement (60%–80% agreed), equipoise (40%–60% agreed) or disagreement (fewer than 40% agreed). A final version of the document was then submitted to all group chairs of the respective societies for approval (Figure 1). A summary of the supporting evidence, all final consensus statements and their levels of evidence and grades (Appendix 2) are presented herein.
Figure 1.
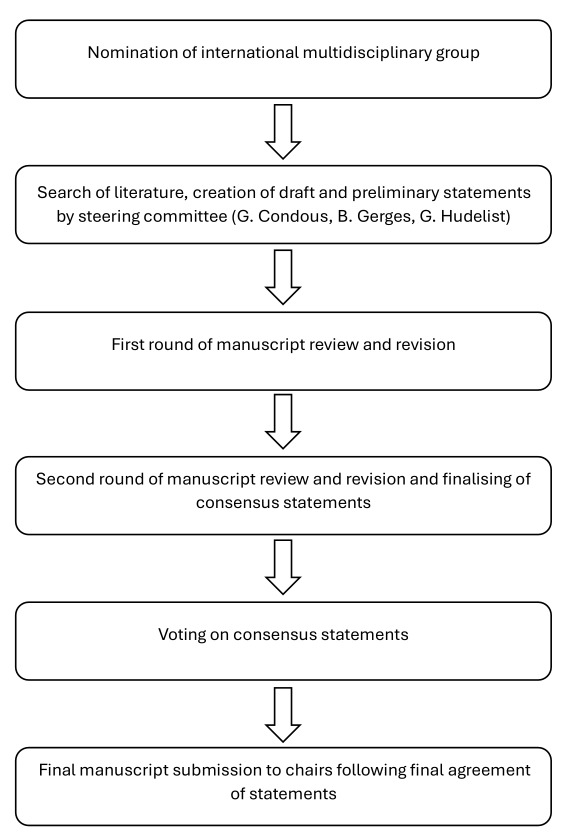
Process for development of Consensus Statement on the use of non-invasive imaging techniques for diagnosis and classification of pelvic deep endometriosis.
Literature review
Transvaginal sonography (TVS)
Rectosigmoid DE
Since Bazot et al. (2004) evaluated the accuracy of TVS against surgical findings of pelvic DE, there have been a considerable number of studies published assessing preoperatively imaging techniques to detect DE, in particular rectosigmoid DE. Of these, TVS is the most studied, and is often used as the first-line modality, given its accessibility, relatively low cost and non-invasiveness (Piessens et al., 2014). In the Cochrane review published in 2016 by Nisenblat et al. (2016), the overall pooled sensitivity and specificity for TVS were 90% and 96%, respectively (14 studies). Noventa et al. (2019) performed a meta-analysis using a head- to-head approach and, on comparison of TVS vs MRI studies, they found the pooled sensitivity of TVS to be 85% and the specificity, based on their data, was 94%. Subsequently, there were two well-conducted meta-analyses, although they each included a small number of studies, specifically eight (Moura et al., 2019) and 11 (Pereira et al., 2020). Moura et al. ( 2019) performed a meta-analysis comparing TVS and MRI in the diagnosis of rectosigmoid DE in the same population, and found TVS to be marginally superior to MRI, with sensitivities of 90% and 88%, respectively, and specificities of 96% and 90%. Pereira et al. (2020) published a comparative study of TVS and MRI, including enhancing techniques, and reported a sensitivity and specificity of 80% and 94%, respectively, for TVS. Most recently Gerges et al. (2021a) performed a systematic review and meta-analysis of prospective studies, limited to those with at least 10 affected and 10 unaffected patients, and found an overall pooled sensitivity of studies assessing TVS for the detection of rectal/rectosigmoid DE (21 studies) of 89%, and specificity of 97%. Furthermore, in their subgroup analyses of 13 studies using two-dimensional (2D) TVS and five studies using TVS with rectal water contrast (RWC), the sensitivities and specificities were similar, at 84% and 97%, respectively, for 2D-TVS, and 88% and 97%, respectively, for TVS-RWC. A comparison of the included meta- analyses for the detection of rectosigmoid DE is summarised in Table I.
Table I.
Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for detection of deep endometriosis of the rectosigmoid.
| Study | Imaging modality | Studies (n) | Patients (n) | Sensitivity | Specificity | LR+ | LR- |
|---|---|---|---|---|---|---|---|
| (Hudelist et al., 2011) | TVS | 10 | 1106 | 0.91 (0.88–0.94) | 0.98 (0.97–0.99) | 30.36 (15.46–59.63) | 0.09 (0.05–0.19) |
| (Medeiros et al., 2015) | MRI | 6 | 611 | 0.83 (0.78–0.87) | 0.88 (0.84–0.92) | 6.92* | 0.19* |
| (Guerriero et al., 2016b) | TVS | 19 | 2639 | 0.91 (0.85–0.94) | 0.97 (0.95–0.98) | 33.6 (17.8–63.5) | 0.11 (0.06–0.21) |
| (Nisenblat et al., 2016) | TVS | 14 | 1616 | 0.90 (0.82–0.97) | 0.96 (0.94–0.99) | 22.50* | 0.10* |
| MRI | 6 | 612 | 0.92 (0.86–0.99) | 0.96 (0.93–0.98) | 23.00* | 0.08* | |
| RES | 4 | 330 | 0.91 (0.85–0.98) | 0.96 (0.91–1.00) | 22.75* | 0.09* | |
| CT | 3 | 389 | 0.98 (0.94–1.00) | 0.99 (0.97–1.00) | 98.00* | 0.02* | |
| DCBE | 2 | 106 | 0.56 (0.32–0.80) | 0.77 (0.41–1.00) | 2.43* | 0.57* | |
| (Guerriero et al., 2018a) | TVS | 6 | 424 | 0.85 (0.68–0.94) | 0.96 (0.85–0.99) | 20.4 (4.7–88.5) | 0.16 (0.07–0.38) |
| MRI | 6 | 424 | 0.85 (0.78–0.90) | 0.95 (0.83–0.99) | 18.4 (4.7–72.4) | 0.16 (0.11–0.24) | |
| (Moura et al., 2019) | TVS | 8 | 1132 | 0.90 (0.87–0.92) | 0.96 (0.94–0.97) | 20.66 (8.71–49.00) | 0.12 (0.08–0.20) |
| MRI | 8 | 1132 | 0.88 (0.85–0.91) | 0.90 (0.88–0.92) | 17.26 (3.57–83.50) | 0.15 (0.10–0.23) | |
| (Noventa et al., 2019) | |||||||
| TVS vs MRI | TVS | 8 | 900 | 0.85 (0.76–0.90) | 0.94* | 14.17* | 0.16* |
| MRI | 8 | 900 | 0.83 (0.76–0.88) | 0.93* | 11.86* | 0.18* | |
| TVS vs RES | TVS | 7 | 710 | 0.89 (0.84–0.93) | 0.95* | 17.80* | 0.12* |
| RES | 7 | 710 | 0.88 (0.84–0.91) | 0.91* | 9.78* | 0.13* | |
| MRI vs RES | MRI | 6 | 842 | 0.84 (0.79–0.88) | 0.91* | 9.33* | 0.18* |
| RES | 6 | 842 | 0.91 (0.87–0.94) | 0.87* | 7.00* | 0.10* | |
| (Pereira et al., 2020) | TVS | 11 | 1362 | 0.80 (0.62–0.91) | 0.94 (0.87–0.97) | 13.7 (5.5–34.2) | 0.21 (0.10–0.44) |
| MRI | 11 | 1362 | 0.82 (0.68–0.91) | 0.94 (0.86–0.97) | 13.1 (5.3–32.5) | 0.19 (0.10–0.38) | |
| (Gerges et al., 2021a) | TVS | 21 | 2857 | 0.89 (0.83–0.92) | 0.97 (0.95–0.98) | 30.8 (17.6–54.1) | 0.12 (0.08–0.17) |
| MRI | 7 | 852 | 0.86 (0.79–0.91) | 0.96 (0.94–0.97) | 21.0 (13.4–33.1) | 0.15 (0.09–0.23) | |
| CT | 6 | 402 | 0.93 (0.84–0.97) | 0.95 (0.81–0.99) | 37.1 (21.1–65.4) | 0.08 (0.05–0.14) | |
| RES | 8 | 850 | 0.92 (0.87–0.95) | 0.98 (0.96–0.99) | 20.3 (4.3–94.9) | 0.07 (0.03–0.19) | |
Only first author of each study is given. Data in parentheses are 95% CI. *Value calculated from available study data. CT, computed tomography; DCBE, double contrast barium enema; LR+, positive likelihood ratio; LR−, negative likelihood ratio; MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultrasound.
Uterosacral ligaments/torus uterinus (USL), rectovaginal septum (RVS) and vaginal DE
Despite the uterosacral ligaments (USL) being one of the most commonly affected sites, DE being found at this location during laparoscopy in up to 61% of patients (Fratelli et al., 2013), assessment by TVS of this location is more challenging than at other sites. The performance of TVS for the preoperative diagnosis of USL DE is similar across several published meta-analyses. Nisenblat et al. (2016) compared TVS, transrectal sonography and MRI imaging modalities and found a sensitivity of 64% and specificity of 97% for the detection of USL DE by TVS, from a total of seven studies. Guerriero et al. (2015; 2018a) published two reviews: the first, Guerriero et al. (2015), assessed TVS and included 11 studies, finding a sensitivity and specificity of 53% and 93%, respectively, whilst, Guerriero et al. (2018a), in a head-to-head review, comparing TVS and MRI, included six studies and found a sensitivity and specificity for TVS of 67% and 86%, respectively. These results were slightly lower than those of the head-to-head review of Noventa et al. (2019) who reported a sensitivity for TVS of 71%, while the specificity calculated from their data was 89%, in the TVS vs MRI analysis, likely due to their inclusion of retrospective studies. The most recent systematic review and meta-analysis, by Gerges et al. (2021b), which included prospective studies that assessed preoperatively any imaging modality for the detection of DE in the USL, rectovaginal septum (RVS) and vagina, correlated with the reference standard of surgical data and/or histology, reported a pooled sensitivity and specificity of TVS for USL of 60% and 95%, respectively.
The performance of TVS for the detection of RVS and vaginal DE was found to be poorer than that of other modalities, particularly when compared to MRI. In the first review by Guerriero et al. (2015), the sensitivity and specificity of TVS for detection of RVS DE were 49% and 98% and those for vaginal DE were 58% and 96%, respectively. The results were similar for RVS DE in the two head-to-head reviews, with Guerriero et al. (2018a) finding a sensitivity and specificity of 59% and 97%, respectively, and Noventa et al. (2019) reporting a sensitivity of 47% and with a specificity of 95% calculated from their data. Most recently, Gerges et al. (2021b) reported overall pooled sensitivities and specificities of 57% and 100%, respectively, for RVS DE (seven studies) and 52% and 98% for vaginal DE (four studies). A comparison of the included meta-analyses for the detection of USL, RVS and vaginal DE are summarised in Tables II–IV.
Table II.
Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for detection of deep endometriosis of the uterosacral ligaments.
| Study | Imaging modality | Studies (n) | Patients (n) | Sensitivity | Specificity | LR+ | LR- |
|---|---|---|---|---|---|---|---|
| (Guerriero et al., 2015) | TVS | 11 | 1482 | 0.53 (0.35–0.70) | 0.93 (0.83–0.97) | 7.8 (3.7–16.4) | 0.51 (0.36–0.71) |
| (Medeiros et al., 2015) | MRI | 11 | 1054 | 0.85 (0.82–0.88) | 0.80 (0.77–0.84) | 4.47* | 0.19* |
| (Nisenblat et al., 2016) | TVS | 7 | 751 | 0.64 (0.50–0.79) | 0.97 (0.93–1.00) | 21.33* | 0.37* |
| MRI | 4 | 199 | 0.86 (0.80–0.92) | 0.84 (0.68–1.00) | 5.38* | 0.17* | |
| RES | 2 | 232 | 0.52 (0.29–0.74) | 0.94 (0.86–1.00) | 8.67* | 0.51* | |
| (Guerriero et al., 2018a) | TVS | 6 | 261 | 0.67 (0.55–0.77) | 0.86 (0.73–0.93) | 4.8 (2.6–9.0) | 0.38 (0.29–0.50) |
| MRI | 6 | 261 | 0.70 (0.55–0.82) | 0.93 (0.87–0.97) | 10.4 (5.1–21.2) | 0.32 (0.20–0.51) | |
| (Noventa et al., 2019) | |||||||
| TVS vs MRI | TVS | 6 | 636 | 0.71 (0.65–0.77) | 0.89* | 6.45* | 0.33* |
| MRI | 6 | 636 | 0.67 (0.54–0.77) | 0.93* | 9.57* | 0.35* | |
| TVS vs RES | TVS | 5 | 576 | 0.75 (0.69–0.70) | 0.84* | 4.69* | 0.30* |
| RES | 5 | 576 | 0.61 (0.43–0.76) | 0.69* | 1.97* | 0.57* | |
| (Gerges et al., 2021b) | TVS | 7 | 108 | 0.60 (0.32–0.82) | 0.95 (0.90–0.98) | 13.2 (8.0–21.8) | 0.42 (0.22–0.82) |
| MRI | 4 | 440 | 0.81 (0.66–0.90) | 0.83 (0.62–0.94) | 4.8 (2.1–11.1) | 0.23 (0.14–0.38) | |
Only first author of each study is given. Data in parentheses are 95% CI. *Value calculated from available study data. LR+, positive likelihood ratio; LR−, negative likelihood ratio; MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultra-sound.
Table IV.
Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for detection of deep endometriosis of the vagina.
| Study | Imaging modality | Studies (n) | Patients (n) | Sensitivity | Specificity | LR+ | LR- |
|---|---|---|---|---|---|---|---|
| (Guerriero et al., 2015) | TVS | 9 | 965 | 0.58 (0.40–0.74) | 0.96 (0.87–0.99) | 15.3 (4.6–51.3) | 0.44 (0.29–0.66) |
| (Medeiros et al., 2015) | MRI | 9 | 1021 | 0.82 (0.76–0.86) | 0.82 (0.76–0.86) | 4.56* | 0.22* |
| (Nisenblat et al., 2016) | TVS | 6 | 679 | 0.57 (0.21–0.94) | 0.99 (0.96–1.00) | 57.00* | 0.43* |
| MRI | 4 | 248 | 0.77 (0.67–0.88) | 0.97 (0.92–1.00) | 25.67* | 0.67* | |
| RES | 2 | 232 | 0.39 (0.08–0.70) | 1.00 (1.00–1.00) | —† | 0.61* | |
| (Gerges et al., 2021b) | TVS | 4 | 451 | 0.52 (0.29–0.74) | 0.98 (0.95–0.99) | 27.1 (12.0–61.4) | 0.49 (0.30–0.80) |
| MRI | 3 | 137 | 0.64 (0.40–0.83) | 0.98 (0.83–0.99) | 27.5 (8.4–90.8) | 0.37 (0.19–0.69) |
Only first author of each study is given. Data in parentheses are 95% CI. *Value calculated from available study data. †Value could not be calculated from available study data. LR+, positive likelihood ratio; LR−, negative likelihood ratio; MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultrasound.
Table III.
Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for detection of deep endometriosis of the rectovaginal septum.
| Study | Imaging modality | Studies (n) | Patients (n) | Sensitivity | Specificity | LR+ | LR- |
|---|---|---|---|---|---|---|---|
| (Guerriero et al., 2015) | TVS | 10 | 1482 | 0.49 (0.36–0.62) | 0.98 (0.95–0.99) | 26.9 (10.2–71.3) | 0.52 (0.40–0.67) |
| (Medeiros et al., 2015) | MRI | 7 | 753 | 0.77 (0.69–0.83) | 0.95 (0.92–0.96) | 15.40* | 0.24* |
| (Nisenblat et al., 2016) | TVS | 10 | 983 | 0.88 (0.82–0.94) | 1.00 (0.98–1.00) | —† | 0.12* |
| MRI | 3 | 288 | 0.81 (0.70–0.93) | 0.86 (0.78–0.95) | 5.79* | 0.22* | |
| RES | 2 | 232 | 0.78 (0.51–1.00) | 0.96 (0.89–1.00) | 19.50* | 0.23* | |
| (Guerriero et al., 2018a) | TVS | 5 | 365 | 0.59 (0.26–0.86) | 0.97 (0.94–0.99) | 23.5 (9.1–60.5) | 0.42 (0.18–0.97) |
| MRI | 5 | 365 | 0.66 (0.51–0.79) | 0.97 (0.89–0.99) | 22.5 (6.7–76.2) | 0.38 (0.23–0.52) | |
| (Noventa et al., 2019) | |||||||
| TVS vs MRI | TVS | 7 | 715 | 0.47 (0.23–0.72) | 0.95* | 9.40* | 0.56* |
| MRI | 7 | 715 | 0.61 (0.48–0.72) | 0.92* | 7.63* | 0.58* | |
| TVS vs RES | TVS | 5 | 574 | 0.39 (0.13–0.73) | 0.95* | 7.80* | 0.64* |
| RES | 5 | 574 | 0.55 (0.22–0.84) | 0.89* | 5.00* | 0.51* | |
| MRI vs RES | MRI | 5 | 601 | 0.55 (0.41–0.67) | 0.94* | 9.17* | 0.48* |
| RES | 5 | 601 | 0.55 (0.22–0.84) | 0.89* | 5.00* | 0.51* | |
| (Gerges et al., 2021b) | TVS | 7 | 1005 | 0.57 (0.30–0.80) | 1.00 (0.92–1.00) | 147.1 (7.5–2895.2) | 0.44 (0.23–0.81) |
Only first author of each study is given. Data in parentheses are 95% CI. *Value calculated from available study data. †Value could not be cal- culated from available study data. LR+, positive likelihood ratio; LR−, negative likelihood ratio; MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultrasound.
Since the publication in 2016 of the IDEA consensus opinion (Guerriero et al., 2016a) regarding the sonographic evaluation of the pelvis in women with suspected endometriosis, there has been further delineation of the anatomical terminology used in diagnostic imaging to define the parametrium, paracervix and USL (Di Giovanni et al., 2022; Mariani et al., 2021; Scioscia et al., 2021). This is of particular significance as parametrial DE can be associated with ureteral stenosis, with associated increased operative risks and the potential need for multidisciplinary surgery. Guerriero et al. (2021) published a systematic review and meta-analysis of the accuracy of TVS for the detection of parametrial DE, which included four studies. The pooled sensitivity was 31% and the specificity was 98%, although a positive result on TVS significantly increased the post-test probability, from 18% to 79%. More recently, in a retrospective review, Roditis et al. (2023) found the sensitivity and specificity for the detection of parametrial DE to be 20.7% and 97.1%, respectively, for TVS, and 36% and 93.8% for MRI.
Bladder DE
DE involving the urinary tract, namely the bladder, ureters and kidneys, is a form of DE affecting between 19% and 53% of women with pelvic DE, but only 1–2% of people affected by endometriosis (Saccardi et al., 2017). Given the low incidence of this manifestation of DE, there are limited systematic reviews assessing the preoperative diagnostic accuracy of imaging for bladder DE. Guerriero et al. (2015) performed a systematic review including prospective and retrospective studies that each had at least 50 participants who underwent TVS prior to surgery and found a pooled sensitivity and specificity of 62% and 100%, respectively. Noventa et al. (2019) performed a systematic review of head-to-head studies, including retrospective studies, with only two studies that compared TVS and transrectal endoscopic sonography (RES). They found, by univariate analysis, diagnostic odds ratios of 4.94 for TVS and 3.13 for RES. In a review of prospective studies which assessed preoperatively any imaging modality for the presence of bladder DE, correlated with the gold standard of surgical data and/or histology as reference, and with at least 10 affected and 10 unaffected patients, Gerges et al. (2021c) found an overall pooled sensitivity for detection of bladder DE of 55% and specificity of 99%, although a meta-analysis could not be performed given the limited number of applicable studies. A comparison of the included meta-analyses for the detection of bladder DE is summarised in Table V.
Table V.
Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for detection of deep endometriosis of the bladder.
| Study | Imaging modality | Studies (n) | Patients (n) | Sensitivity | Specificity | LR+ | LR- |
|---|---|---|---|---|---|---|---|
| (Guerriero et al., 2015) | TVS | 8 | 1248 | 0.62 (0.40–0.80) | 1.00 (0.97–1.00) | 208.4 (21.0–2066.0) | 0.38 (0.22–0.66) |
| (Medeiros et al., 2015) | MRI | 5 | 586 | 0.64 (0.48–0.77) | 0.98 (0.96–0.99) | 31.00* | 0.37* |
| (Gerges et al., 2021c) | TVS | 8 | 1052 | 0.55 (0.28–0.79) | 0.99 (0.98–1.00) | 54.5 (18.9–157.4) | 0.46 (0.25–0.85) |
Only first author of each study is given. Data in parentheses are 95% CI. *Value calculated from available study data. LR+, positive likelihood ratio; LR−, negative likelihood ratio; MRI, magnetic resonance imaging; TVS, transvaginal ultrasound.
Magnetic resonance imaging (MRI)
Rectosigmoid DE
The 2016 Cochrane review of Nisenblat et al. (2016) reported an overall sensitivity and specificity for MRI of 92% and 96%, respectively (six studies). More recently, Noventa et al. (2019) performed a meta-analysis using a head-to-head approach and found a pooled sensitivity for MRI of 83%, with a specificity calculated from their data of 93%, when compared with TVS (at 85% and 94%) and 84% and 91%, respectively, when compared with RES (at 91% and 87%). Moura et al. (2019) performed a meta-analysis comparing MRI vs TVS in the diagnosis of rectosigmoid DE in the same population. Both modalities were found to have similar sensitivities (88% vs 90%) and specificities (90% vs 96%). Pereira et al. (2020) published a comparative study of MRI vs TVS, including enhancing techniques, and reported sensitivities of 82% vs 80% and specificities of 94% vs 94%. However, the latter two meta-analyses (Moura et al., 2019; Pereira et al., 2020), although well conducted, each included a small number of studies: eight and 11, respectively. More recently, Gerges et al. (2021a) performed a systematic review and meta-analysis of prospective studies, limited to those with at least 10 affected and 10 unaffected patients, and found the overall pooled sensitivity and specificity of all studies assessing MRI (seven studies, 852 patients) to be 86% and 96%, respectively, whilst the subgroup analysis of 2D- MRI (five studies, 813 patients) had similar results, with a sensitivity and specificity of 85% and 96%, respectively. Due to the limited number of studies, other subgroup analyses were not performed. In a study assessing interobserver agreement, three- dimensional (3D) MRI performed similarly to 2D- MRI for the detection of rectosigmoid DE, with sensitivities for radiologists interpreting 3D-MRI ranging from 89% to 100% and specificities from 94% to 100% (Bazot et al., 2013), while, in another study, MRI with rectal ultrasound gel outperformed 2D-MRI, with a sensitivity of 99% and specificity of 96%, compared with 85% and 96%, respectively (Hottat et al., 2009). A comparison of the included meta-analyses for the detection of rectosigmoid DE is summarised in Table I.
Uterosacral ligaments/torus uterinus (USL), rectovaginal septum (RVS) and vaginal DE
MRI generally outperforms TVS for the detection of USL DE, especially with respect to sensitivity. Nisenblat et al. (2016) compared imaging modalities and found a sensitivity and specificity for the detection of USL DE for MRI (four studies) of 86% and 84%, respectively, compared with 64% and 97% for TVS (seven studies). In the head-to-head review by Guerriero et al. (2018a), from a total of six studies, the sensitivity and specificity, respectively, for the detection of USL DE by MRI were 70% and 93%, compared with 67% and 86% for TVS. Similarly, for RVS DE, the sensitivity and specificity for MRI were 66% and 97%, respectively, compared with 59% and 97% for TVS. In contrast, Noventa et al. (2019) performed a head-to-head meta-analysis including retrospective studies and found TVS to be slightly superior to MRI for the detection of USL DE, with sensitivities of 71% vs 67% and specificities, based on their data, of 89% vs 93%. In contrast, the reported sensitivities, and calculated specificities for the detection of RVS DE were 47% and 95%, respectively, for TVS and 61% and 92% for MRI. In a meta-analysis assessing the performance of MRI in detecting DE, Medeiros et al. (2015) reported sensitivities and specificities for USL DE of 85% and 80%, for RVS DE of 77% and 95% and for vaginal DE of 82% and 82%, respectively. Similarly, the meta-analysis of prospective studies by Gerges et al. (2021b) found MRI to outperform TVS consistently, with sensitivities and specificities for USL DE of 81% and 83%, respectively, for MRI and 60% and 95% for TVS, and sensitivities and specificities for vaginal DE of 64% and 98%, respectively, for MRI and 52% and 98% for TVS. A comparison of the included meta-analyses for the detection of USL, RVS and vaginal DE are summarised in Tables II–IV.
Bladder DE
Studies assessing the diagnostic accuracy of imaging techniques for bladder DE are quite limited in number, largely due to the low incidence of the disease. Medeiros et al. (2015) performed a pooled analysis, including both retrospective and prospective studies, of the detection of bladder DE using MRI. They found a pooled sensitivity and specificity of 64% and 98%, respectively. In a review of prospective studies (Gerges et al., 2021c), while pooled analyses could not be performed due to the limited number of studies, two studies were described which assessed 2D-MRI, reporting sensitivities ranging from 50% (Guerriero et al., 2018b) to 100% (Alborzi et al., 2018) and specificities ranging from 97% (Guerriero et al., 2018b) to 100% (Alborzi et al., 2018). MRI with rectal ultrasound gel performed similarly to this, with a sensitivity of 70% and specificity of 100% (Hottat et al., 2009). A comparison of the included meta-analyses for the detection of bladder DE is summarized in Table V.
Computed tomography (CT)
The use of CT for the preoperative detection of endometriosis is less well studied compared with TVS and MRI, and mostly it is used for detection of rectosigmoid DE. In the 2021 systematic review by Gerges et al. (2021a), six studies were included which assessed CT (402 patients), of which three assessed standard CT (Biscaldi et al., 2007; Ferrero et al., 2011; Stabile Ianora et al., 2013) and three assessed CT colonography (Baggio et al., 2016; Barra et al., 2020; Ferrero et al., 2017). The overall pooled sensitivity and specificity of CT for the detection of rectosigmoid DE were 93% and 95%, respectively (Gerges et al., 2021a). Subanalyses of CT colonography were not performed, and these results ranged widely, with one study(Baggio et al., 2016) finding poor performance, with a sensitivity of 68% and specificity of 67%, while the other two studies reported sensitivities of 93% (Ferrero et al., 2017) and 95% (Barra et al., 2020) and specificities of 87% (Ferrero et al., 2017) and 93% (Barra et al., 2020). The review by Nisenblat et al. (2016) reported better results when CT was combined with water enema, with three studies (389 patients) (Baggio et al., 2016; Ferrero et al., 2011; Stabile Ianora et al., 2013) included, resulting in a pooled sensitivity and specificity of 98% and 99%, respectively. However, Nisenblat et al. (2016) stated that this technique should be avoided in young patients whenever possible, due to the associated radiation exposure (Biscaldi et al., 2021). This is consistent with the ALARA principle, i.e. ensuring that the exposure to radiation is ‘as low as reasonably achievable’ (Hendee and Edwards, 1986).
General remarks on imaging
The test performance of any imaging technique is operator-dependent and will increase with increasing levels of training, skills and experience of the operator. Also, as systematic reviews, by definition, include older studies, and because expertise in imaging of endometriosis has improved dramatically worldwide in the last few years, it is reasonable to assume that the published sensitivity figures are an underestimation of the current status. The following statements should be interpreted based on these assumptions. Also, whilst, herein, these imaging techniques have been compared with each other in various anatomical areas, they can be complementary and do not need to be used exclusively (Bielen et al., 2020). For example, a recent analysis of the combined use of vaginal palpation, TVS and MRI found that at least two positive tests was the most valid model for diagnosing DE, with an accuracy of 91.4% (Roditis et al., 2023).
Non-invasive use of classification and scoring systems for endometriosis
A multitude of classification and scoring systems for topographical description and expression of the extent of endometriosis and associated secondary adhesions have been proposed and in use for decades, with varying rates of recognition amongst clinicians, radiologists, sonographers and gynaecological surgeons (International working group of AAGL et al., 2021). These include the #Enzian, AAGL classification, EFI, deep Pelvic Endometriosis Index (dPEI), revised American Society of Reproductive Medicine (rASRM) score and Ultrasound-Based Endometriosis Staging System (UBESS).
TVS for description and classification of DE
Terms and definitions for uniform description of DE with ultrasound standardised across different centres and countries have been proposed by the IDEA group and are now widely accepted (Guerriero et al., 2016a). These definitions serve primarily as standardised terminology for describing DE with ultrasound. Their use, applicability, accuracy and reproducibility are currently under investigation in an international multicentre study (IDEA Phase 1). As part of this, Leonardi et al. (2022) recently published the results of a pilot study on the accuracy of the IDEA terms and definitions for presurgical detection of DE. This included 273 women with suspected endometriosis, of whom 256 (93.8%) had endometriosis confirmed, of which 190 (74.2%) were DE cases. In these women, the diagnostic accuracy of TVS using IDEA definitions was 86.1%, sensitivity was 88.4%, specificity was 78.8%, positive predictive value (PPV) was 92.9%, negative predictive value (NPV) was 68.4%, positive likelihood ratio (LR+) was 4.17 and negative likelihood ratio (LR−) was 0.15. Applying the IDEA criteria in 537 women with suspected endometriosis, Szabo et al. (2024) demonstrated a diagnostic accuracy for TVS in the diagnosis of colorectal DE of 94%, sensitivity of 93.5%, specificity of 94.6%, NPV of 93.1%, PPV of 94.9%, LR+ of 17.24 and LR− of 0.07.
Amongst all scoring and/or classification systems for endometriosis published so far, the rASRM score (1997) (Figure S1), the #Enzian classification (Keckstein et al., 2021; Keckstein et al., 2003) (Figure S2), the UBESS (Menakaya et al., 2016) (Figure S3), the EFI for prediction of conception following surgery for endometriosis(Adamson and Pasta, 2010, Tomassetti et al., 2021) (Figure S4) and the AAGL endometriosis classification (Abrao et al., 2021) have also been investigated for their non-invasive applicability using TVS and/ or MRI. Ideally, it should be possible to describe endometriosis via scoring and classification systems common to all, including surgeons, radiologists and sonographers, to facilitate communication and clinical research.
The rASRM score defines degrees of severity of endometriosis in four stages (minimal (Stage I), mild (Stage II), moderate (Stage III) and severe (Stage IV)), based on endometriotic lesions affecting the pelvic peritoneum, ovaries and associated adhesions. Points are allocated according to whether the lesion is deep or superficial, the lesion size, and the type (filmy or dense) and extent of adhesions involving the Fallopian tubes, ovaries and pouch of Douglas, and are combined to give a total score that corresponds to one of the four possible stages. Leonardi et al. (2020) investigated retrospectively the accuracy of TVS for staging endometriosis preoperatively in 204 patients using the rASRM classification. When evaluating the stages separately, the sensitivity, specificity, PPV and NPV of TVS were 18.2%, 94.7%, 80.0% and 49.7%, respectively, for rASRM Stage I; 22.7%, 96.7%, 45.5% and 91.2% for Stage II; 62.5%, 92.0%, 40.0% and 96.7% for Stage III; and 71.9%, 97.1%, 82.1% and 94.9% for Stage IV. Similar to this observation of Leonardi et al. (2020) that TVS had lower accuracy on assessment in minimal and mild rASRM stages of disease, Holland et al. (2010) found low sensitivity of TVS for diagnosing minimal and mild endometriosis but an accuracy of 94% for detection of moderate and severe disease. Of note, both authors observed low diagnostic accuracy for TVS in the detailed assessment of DE, due to the fact that DE could not be scored clearly using the rASRM classification. Finally, Tomassetti et al. (2021) found good agreement with findings at laparoscopy using TVS for estimating the EFI, which is based partly on the rASRM. So far, there have been no attempts to use MRI in combination with the rASRM score to describe and diagnose endometriosis.
To improve classification of DE, the Enzian system was developed in 2003 (Keckstein et al., 2003) and further extended and modified in 2021 (Keckstein et al., 2021). Five studies have evaluated the accuracy of TVS in combination with the Enzian classification. Hudelist et al. (2021b) compared TVS findings with surgical findings in 195 women with DE and found good agreement between these modalities, especially for Enzian compartments A (vagina, rectovaginal space, retrocervical area), C (rectum) and FB (urinary bladder). TVS detected DE in compartments A, B (USL, cardinal ligaments, pelvic sidewall), C and FB with sensitivities of 84%, 91%, 92% and 88%, respectively, and specificities of 85%, 73%, 95% and 99%. Recently, Enzelsberger et al. (2022) evaluated preoperative use of the Enzian classification using TVS and/or MRI in a prospective multicentre study including 1062 women undergoing surgery for endometriosis, and observed lower accuracy, compared with laparoscopic evaluation, for TVS and/or MRI for Enzian compartments A, B and C. Complete concordance between compartment and imaging Grade 1, 2 or 3 was observed in 369 women (35.14% of 1050 valid ratings), which increased to 40.3% when the numerical ratings in compartments A/B/C were categorized into ‘affected’ (combining Grades 1, 2 and 3) and ‘not affected’ (coded as 0). Overall concordance, sensitivity, specificity, PPV and NPV, respectively, of TVS and/or MRI relative to surgical evaluation for compartment A were 83%, 63%, 91%, 72% and 88%, for compartment B were 69%, 47%, 86%, 72% and 68%, and for compartment C were 89%, 52%, 96%, 76% and 91%. However, either MRI or TVS could be applied and, also, TVS was performed by sonographers with limited experience in scanning DE, which limits the conclusions that can be drawn from these results regarding the accuracy of TVS when used in combination with the Enzian classification.
#Enzian.
In order to test the accuracy of the modified Enzian classification, the so-called #Enzian classification, which also takes into account peritoneal and ovarian endometriosis and secondary tubal adhesions, and has been shown to outperform the rASRM score regarding description of the extent of DE (Montanari et al., 2022a), Di Giovanni et al. (2023) investigated retrospectively using the #Enzian classification 93 patients who had undergone TVS prior to surgery. They found sensitivities and specificities of TVS in the identification of endometriosis in compartment O (ovary) of 100% and 100%, respectively (right) and 100% and 96% (left), compartment A of 97% and 86%, compartment B of 100% and 90% (right) and 97% and 70% (left), compartment C of 100% and 96%, compartment FB of 86% and 100%, compartment FI (intestinum) of 100% and 100%, and compartment FU (ureters) of 100% and 100%. Bindra et al. (2023) reviewed retrospectively 50 patients undergoing surgery following TVS mapping used with #Enzian and observed accuracy values similar to those reported by Di Giovanni et al. (2023). Recently, Montanari et al. (2022b) evaluated the #Enzian classification in a prospective, multicentre study, including 745 patients undergoing TVS and surgery for DE. The sensitivity for detection of endometriotic lesions ranged from 50% (#Enzian compartment FI) to 95% (#Enzian A) and specificity ranged from 86% (#Enzian T (tubo- ovarian condition), left) to 99% (#Enzian FI) or 100% (#Enzian FB (urinary bladder), #Enzian FU and #Enzian FO (other extragenital locations)), with PPVs ranging from 90% (#Enzian T, right) to 100% (#Enzian FO), NPVs ranging from 74% (#Enzian B, left) to 99% (#Enzian FB and #Enzian FU) and accuracy ranging from 88% (#Enzian B, right) to 99% (#Enzian FB), confirming that the presence and extent of DE can be evaluated accurately using TVS in combination with the #Enzian classification.
UBESS.
The UBESS was created in order to stage disease extent and predict the complexity of surgery in patients with DE, based on the anatomical location of DE and sonographic markers of local invasiveness (Menakaya et al., 2016). In a multicentre prospective and retrospective cohort study including 192 consecutive women with suspected endometriosis, three stages of UBESS (I–III) were correlated with three levels of complexity of laparoscopic surgery. The accuracy of UBESS Stage III in predicting the need for advanced laparoscopic surgery was 95.3%, sensitivity was 94.8%, specificity was 95.5%, PPV was 90.2%, NPV was 97.7%, LR+ was 21.2 and LR− was 0.054 (Menakaya et al., 2016). External validation of the UBESS showed it to have little predictive value for surgical difficulty in a small proportion of 33 patients (Chaabane et al., 2019) and revealed problems with generalizability to cases lacking bowel DE or lacking obliteration of the pouch of Douglas (Espada et al., 2021).
AAGL classification and EFI.
Amongst other systems for classification and scoring of endometriosis that have been proposed (International working group of AAGL et al., 2021) is the ultrasound-based 2021 AAGL endometriosis classification (Abrao et al., 2021). This system was evaluated recently by Abrao et al. (2023), who showed that it is only accurate in AAGL Stages I and IV and distinguishes reliably AAGL Stages I–II from Stages III–IV. They found that ultrasound best identified endometriosis of the ovaries, bladder and bowel, but was more limited for the Fallopian tubes and superficial peritoneum. The EFI works primarily as a model to predict fertility outcome following surgery for endometriosis. It constitutes a 10-point scoring system based on factors such as patient characteristics (age, duration of infertility and history of prior pregnancy), the rASRM classification and functionality of Fallopian tubes and ovaries during surgery. One study has demonstrated the possibility of applying the EFI with ultrasound instead of invasive methods, showing that the prediction model can be assessed using TVS-based tubal patency testing, with a 10% loss of accuracy compared with the invasive application of EFI5.
MRI for description and classification of DE
Two consensus MRI lexicons from the Society of Abdominal Radiology (SAR)(Jha et al., 2020) and from the French Society of Women’s Imaging (SIFEM) (Rousset et al., 2023) were published recently. They both describe the different locations of DE according to a compartment-based approach of the pelvis. The most recent one (Rousset et al., 2023) emphasised the description of lateral compartments, which are usually difficult to detect with TVS and are crucial for surgical planning.
Several studies have investigated use of the Enzian classification in conjunction with MRI, reporting good agreement rates between radiological and surgical findings except for B-compartment lesions (Burla et al., 2019; Di Paola et al., 2015; Fendal Tunca et al., 2023; Widschwendter et al., 2022). Manganaro et al. (2021) and Burla et al. (2021) showed that the Enzian classification based on MRI findings is also reproducible. In addition, Thomassin-Naggara et al. (2020) demonstrated that, for DE lesions in compartments A and C, using MRI in conjunction with Enzian classification was accurate in predicting operating time, hospital stay and postoperative complications according to the Clavien–Dindo classification. However, they highlighted the poor reproducibility of the description of B-compartment lesions due to the difficulty of measuring USL on MRI. The same limitation was noted in a recent prospective international multicentre study performed in 12 centres (1062 women) (Enzelsberger et al., 2022), which demonstrated that MRI-based and surgical Enzian classifications were concordant for DE lesions in compartment A in 78.7% (118/150) of cases and compartment C in 82.7% (124/150) of cases, but only in 34.7% (52/150) of cases with lesions in compartment B. Another MRI classification was published in 2020 (Thomassin- Naggara et al., 2020), the dPEI classification, which demonstrated high reproducibility (kappa = 0.74), including for the USL (Figure S5). This MRI classification includes description of lateral compartments and predicts accurately operating time, hospital stay and postoperative complications (Thomassin-Naggara et al., 2023). Larger prospective European and American validation studies on the use of MRI-based #Enzian and dPEI classifications are ongoing.
Consensus Statements
General statements
-
The test performance of any imaging technique for the detection of DE is operator-dependent and will increase with exposure, level of training and skills and experience of the operator.
Consensus: yes, 96.2% (n = 51); no, 0% (n = 0); abstain, 3.8% (n = 2)
-
Patients with a plan for surgical intervention for endometriosis should undergo preoperative imaging for the detection of DE performed by adequately trained operators.
Consensus: yes, 96.2% (n = 51); no, 0% (n = 0); abstain, 3.8% (n = 2)
-
TVS performed by adequately trained operators is recommended as the first-line imaging tool due to its availability, good test performance, cost efficacy and its low environmental impact when compared to other imaging methods.
Level of evidence: 1a
Grade of statement: A
Consensus: yes, 96.2% (n = 51); no, 0% (n = 0); abstain, 3.8% (n = 2).
Statements on ultrasonography
-
Imaging with TVS can reliably preoperatively predict, and is recommended to detect, the presence of DE of the rectum, but is less accurate in predicting sigmoidal DE due to limited visibility.
Level of evidence: 1a
Grade of statement: A
Consensus: yes, 86.8% (n = 46); no, 5.7% (n = 3); abstain, 7.5% (n = 4)
-
Imaging with TVS can help to preoperatively predict the presence of DE of the RVS.
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 83.0% (n = 44); no, 3.8% (n = 2); abstain, 13.2% (n = 7)
-
Imaging with TVS can help to preoperatively predict the presence of DE of the vagina, USL and parametrium.
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 73.6% (n = 39); no, 18.9% (n = 10); abstain, 7.5% (n = 4)
-
Imaging with TVS can help to preoperatively predict the presence of DE of the bladder.
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 90.6% (n = 48); no, 1.9% (n = 1); abstain, 7.5% (n = 4).
Statements on MRI and CT
-
Imaging with MRI can reliably preoperatively predict the presence of DE of the rectosigmoid.
Level of evidence: 1a
Grade of statement: A
Consensus: yes, 90.6% (n = 48); no, 5.7% (n = 3); abstain, 3.8% (n = 2)
-
Imaging with MRI can reliably preoperatively predict the presence of DE of the USL and torus uterinus.
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 88.7% (n = 47); no, 0% (n = 0); abstain, 11.3% (n = 6)
-
Imaging with MRI is helpful to preoperatively predict the presence of DE of the RVS.
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 90.6% (n = 48); no, 3.8% (n = 2); abstain, 5.7% (n = 3)
-
Imaging with MRI can reliably preoperatively predict the presence of DE of the vagina.
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 86.8% (n = 46); no, 3.8% (n = 2); abstain, 9.4% (n = 5)
-
Imaging with MRI can reliably preoperatively predict the presence of DE of the bladder.
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 92.5% (n = 49); no, 3.8% (n = 2); abstain, 3.8% (n = 2)
-
Imaging with CT may reliably preoperatively predict the presence of DE of the rectosigmoid but is less studied than other imaging modalities. There are, however, no obvious advantages compared to MRI, as well as the disadvantage of radiation exposure.
Level of evidence: 2a
Grade of statement: B
Consensus: yes, 69.8% (n = 37); no, 22.6% (n = 12); abstain, 7.5% (n = 4)
-
There is insufficient evidence to support, compared to other imaging modalities, the use of CT for the detection of DE of the USL, torus uterinus, RVS, vagina or bladder.
Level of evidence: 2a
Grade of statement: D
Consensus: yes, 90.6% (n = 48); no, 1.9% (n = 1); abstain, 7.5% (n = 4).
Statements on the non-invasive use of classification systems
-
Imaging with TVS in combination with the rASRM score can help to describe moderate to severe endometriosis but is less accurate in cases of minimal to mild disease as classified with the rASRM score.
Level of evidence: 4
Grade of statement: D
Consensus: yes, 62.3% (n = 33); no, 7.5% (n = 4); abstain, 30.2% (n = 16)
-
Imaging with TVS in combination with the #Enzian classification can reliably describe DE, ovarian endometriosis and adhesions, but is less accurate in cases of parametrial involvement (compartment B).
Level of evidence: 1a
Grade of statement: B
Consensus: yes, 83.0% (n = 44); no, 3.8% (n = 2); abstain, 13.2% (n = 7)
-
Imaging with MRI in combination with the #Enzian classification can reliably describe rectal and RVS DE and ovarian endometriosis but is less accurate in cases of USL and/or parametrial involvement (compartment B) and adhesions.
Level of evidence: 4
Grade of statement: B
Consensus: yes, 81.1% (n = 43); no, 5.7% (n = 3); abstain, 13.2% (n = 7)
-
Imaging with TVS in combination with the UBESS classification may help to estimate surgical complexity, but the predictive value is not yet generalizable.
Level of evidence: 3b
Grade of statement: B
Consensus: yes, 64.2% (n = 34); no, 5.7% (n = 3); abstain, 30.2% (n = 16)
-
Imaging alone with TVS and in combination with the EFI prediction cannot be used reliably as a substitute for the EFI generated by invasive, i.e. surgical, methods.
Level of evidence: 4
Grade of statement: D
Consensus: yes, 62.3% (n = 33); no, 7.5% (n = 4); abstain, 30.2% (n = 16)
-
Imaging alone with TVS in combination with the AAGL classification may be used as a substitute for the AAGL classification generated by invasive, i.e. surgical, methods.
Level of evidence: 2b
Grade of statement: C
Consensus: yes, 50.9% (n = 27); no, 28.3% (n = 15); abstain, 20.8% (n = 11).
Discussion
The present work represents a Consensus Statement regarding the use of non-invasive imaging methods, particularly TVS and MRI, in the application of classification systems for the detection of DE. The test performance of any imaging technique is operator-dependent. Imaging with TVS and MRI needs to be performed by well-trained medical staff. TVS is recommended as a first-line imaging tool, due to its availability, good test performance, cost efficacy and low environmental impact. However, it is acknowledged that many centres adopt MRI as a first-line technique, which is also appropriate.
There was strong agreement that TVS assessment of patients with suspected DE will determine accurately or rule out the presence of DE affecting the rectum, RVS and bladder, but that TVS is less precise in locations such as the parametrium and the USL. However, the detection of DE of the USL and parametrium using TVS is evolving and constantly improving. MRI-based imaging is capable of detecting DE in these locations and a consensus was reached that MRI can reliably predict the presence of USL, parametrial and RVS DE.
The use of classification systems for DE is a matter of ongoing debate. There was moderate agreement regarding the non-invasive use of rASRM and UBESS classification systems and the EFI prediction model, and equipoise regarding the usefulness of TVS-based use of the AAGL classification. The majority of participants agreed strongly on the use of TVS or MRI in combination with the #Enzian classification, although it is less accurate in cases of parametrial and USL involvement. Future studies on rASRM, AAGL, UBESS, EFI and #Enzian classification will hopefully further clarify their role in the setting of parametrial and USL involvement.
It is noteworthy that the reference standards in many published studies were laparoscopy, with or without histopathology. Hence, it is difficult to ascertain the limitation of operator expertise, or a reference standard which could be used in women who are managed conservatively. While this Statement focused on non-invasive imaging primarily for planning surgery, this is not the only aspect of endometriosis treatment, because at least 40% of women with DE are asymptomatic. Furthermore, in those with symptoms, it is not always clear that these are caused by or coincide with endometriosis. The statements herein pertain primarily to women with symptomatic disease with a possible plan for surgical treatment. Assessment of women with potential DE by means of non- invasive imaging with TVS and/or MRI performed by appropriately trained clinicians, combined with planning of surgical and/or conservative management approaches, should be the standard of care in healthcare facilities offering endometriosis therapy.
Supporting information online:
The following supporting information may be found in the online version of this article:
Revised American Society for Reproductive Medicine (rASRM) classification of endometriosis. Reprinted from the Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21. Copyright© 1997 American Society for Reproductive Medicine, with permission from Elsevier. All rights reserved.
#Enzian classification system for women with superficial, ovarian and deep endometriosis. Reprinted from Keckstein et al. (2021), with permission from J. Keckstein. Copyright© 2021 The Authors. Published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG). Sacrouterine ligg/USL, uterosacral ligaments.
Ultrasound-based Endometriosis Staging System (UBESS), with sonographic features demonstrable on transvaginal ultrasound (TVS) and its prediction of level of surgical complexity. Adapted from Menakaya et al. (2016), with permission from ISUOG. SVG, sonovaginography.
Endometriosis fertility index (EFI) system. This score predicts fertility outcome for women who attempt non-in-vitro fertilization conception following surgically documented endometriosis. Reprinted from Adamson and Pasta, 2010. Copyright© 2010 American Society for Reproductive Medicine, with permission from Elsevier. All rights reserved. AFS, American Fertility Society.
Magnetic resonance imaging (MRI) lexicon and deep pelvic endometriosis index (dPEI) classification: low extension (score 1 or 2), moderate extension (score 3 or 4) or severe extension (score 5 or more). Reproduced from Rousset et al. (2023). Copyright© 2022 The Author(s). Published by Elsevier Masson SAS on behalf of Société française de radiologie. All rights reserved.
Appendix 1: Search strategy used for identification of potentially relevant studies with corresponding number of studies found (literature search using MEDLINE)
Appendix 2: Levels of evidence and grades of statement used in this work(Guyatt et al., 2008; Howick et al., 2011).
Footnotes
Initiated through ISUOG, the decision to develop this multidisciplinary Consensus Statement was made jointly by ISUOG, ESGE, EEL, ESHRE, AAGL, ISGE, ESUR and IDEA. ISUOG provided administrative support. ISUOG, ESGE, EEL, ESHRE, AAGL, ISGE, ESUR and IDEA are non- profit-making professional societies.
The development group (including all authors) is responsible collectively for the decision to submit for publication. George Condous (chair), Gernot Hudelist (chair) and Bassem Gerges (methodologist) wrote the first draft of the manuscript. All other contributors actively gave personal input, reviewed the manuscript and gave final approval before submission.
Citation: This Consensus Statement should be cited as: ‘Condous G, Gerges B, Thomassin-Naggara I, Becker C, Tomassetti C, Krentel H, van Herendael BJ, Malzoni M, Abrao MS, Saridogan E, Keckstein J, Hudelist G and Collaborators. Non-invasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: an International Consensus Statement. Facts, Views and Vision in ObGyn 2024. DOI: doi.org/10.52054/FVVO.16.2.012.
Acknowledgements: This paper has been simultaneously co-published in Ultrasound in Obstetrics & Gynecology, Facts, Views and Vision in ObGyn, The Trocar, Human Reproduction Open, Journal of Minimally Invasive Gynecology and European Journal of Radiology, by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), the European Society for Gynaecological Endoscopy (ESGE), the European Endometriosis League (EEL), the European Society of Human Reproduction and Embryology (ESHRE), the International Society forGynecologic Endoscopy (ISGE), the American Association of Gynecologic Laparoscopists (AAGL), the International Deep Endometriosis Analysis (IDEA) group, the European Society of Urogenital Radiology (ESUR) and Elsevier B.V.
The authors thank AAGL, EEL, ESGE, ESHRE, ESUR, IDEA, ISGE and ISUOG, and their chairs, for their support. This Consensus Statement has been endorsed by Endometriosis Australia, the Australasian Gynaecological Endoscopy & Surgery (AGES) Society, the Society of Endometriosis and Uterine Disorders (SEUD), Stiftung Endometrioseforschung (SEF) and Arbeitsgemeinschaft Gynäkologische Endoskopie (AGE) Germany. The coordinating chairs (G.C., G.H.) also wish to express sincere gratitude to Hayley Moffitt for organising the teleconferences.
Conflict of interest: TG.C. declares receipt of an MRFF grant to develop AI for endometriosis imaging, honoraria from Samsung and GE healthcare, is a member of the ISUOG Board of trustees, and is the WFUMB President-Elect. E.S. declares grants from National Institute for Health Research, Rosetrees Trust and Barts and the London Charity for trials on endometriosis and hysterectomy, book royalties (for the book entitled ‘Minimally Invasive Surgery in Gynecological Practice’) from De Gruyter, consulting fees from Hologic; honoraria from Medtronic, Hologic, Karl Storz, Intuitive, and Olympus; and was a member of the Medicines for Women’s Health Expert Advisory Group of the Medicines and Healthcare Products Regulatory Agency (MHRA). The other authors do not have any conflicts of interest to declare.
Contributor Information
Intersociety Consensus Group:
K Aas-Eng, J.L. Alcazar, C Bafort, M Bazot, D Bielen, A Bokor, T Bourne, F Carmona, A Di Giovanni, D Djokovic, A Egekvist, J English, C Exacoustos, H Ferreira, S Ferrero, R Forstner, S Freeman, M Goncalves, G Grimbizis, A Guerra, S Guerriero, F.W. Jansen, D Jurkovic, S Khazali, M Leonardi, C Maciel, L Manganaro, M Mueller, M Nisolle, G Noe, S Reid, H Roman, P Rousset, M Seyer Hansen, S Singh, V Thomas, D Timmerman, U.A. Ulrich, T Van den Bosch, D Van Schoubroeck, and A Wattiez
References
- 1.Abrao MS, Andres MP, Gingold JA, et al. Preoperative Ultrasound Scoring of Endometriosis by AAGL 2021 Endometriosis Classification Is Concordant with Laparoscopic Surgical Findings and Distinguishes Early from Advanced Stages. J Minim Invasive Gynecol. 2023;30:363–373. doi: 10.1016/j.jmig.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Abrao MS, Andres MP, Miller CE, et al. AAGL 2021 Endometriosis Classification: An Anatomy-based Surgical Complexity Score. J Minim Invasive Gynecol. 2021;28:1941–1950.:e1. doi: 10.1016/j.jmig.2021.09.709. [DOI] [PubMed] [Google Scholar]
- 3.Abrao MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329–339. doi: 10.1093/humupd/dmv003. [DOI] [PubMed] [Google Scholar]
- 4.Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94:1609–1615. doi: 10.1016/j.fertnstert.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal SK, Chapron C, Giudice LC, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220:354.:e1–12. doi: 10.1016/j.ajog.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Alborzi S, Rasekhi A, Shomali Z, et al. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine (Baltimore) 2018;97:e9536. doi: 10.1097/MD.0000000000009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baggio S, Zecchin A, Pomini P, et al. The Role of Computed Tomography Colonography in Detecting Bowel Involvement in Women With Deep Infiltrating Endometriosis: Comparison With Clinical History, Serum Ca125, and Transvaginal Sonography. J Comput Assist Tomogr. 2016;40:886–891. doi: 10.1097/RCT.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 8.Barra F, Biscaldi E, Scala C, et al. A Prospective Study Comparing Three-Dimensional Rectal Water Contrast Transvaginal Ultrasonography and Computed Tomographic Colonography in the Diagnosis of Rectosigmoid Endometriosis. Diagnostics (Basel) 2020;10:252. doi: 10.3390/diagnostics10040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazot M, Stivalet A, Darai E, et al. Comparison of 3D and 2D FSE T2-weighted MRI in the diagnosis of deep pelvic endometriosis: preliminary results. Clin Radiol. 2013;68:47–54. doi: 10.1016/j.crad.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Bazot M, Thomassin I, Hourani R, et al. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obstet Gynecol. 2004;24:180–185. doi: 10.1002/uog.1108. [DOI] [PubMed] [Google Scholar]
- 11.Becker CM, Bokor A, Heikinheimo O, et al. ESHRE guideline: endometriosis. Hum Reprod Open. 2022;2022:hoac009. doi: 10.1093/hropen/hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendifallah S, Roman H, Rubod C, et al. Impact of hospital and surgeon case volume on morbidity in colorectal endometriosis management: a plea to define criteria for expert centers. Surg Endosc. 2018;32:2003–2011. doi: 10.1007/s00464-017-5896-z. [DOI] [PubMed] [Google Scholar]
- 13.Bielen D, Tomassetti C, Van Schoubroeck D, et al. IDEAL study: magnetic resonance imaging for suspected deep endometriosis assessment prior to laparoscopy is as reliable as radiological imaging as a complement to transvaginal ultrasonography. Ultrasound Obstet Gynecol. 2020;56:255–266. doi: 10.1002/uog.21868. [DOI] [PubMed] [Google Scholar]
- 14.Bindra V, Madhavi N, Mohanty GS, et al. Pre-operative mapping and structured reporting of pelvic endometriotic lesions on dynamic ultrasound and its correlation on laparoscopy using the #ENZIAN classification. Arch Gynecol Obstet. 2023;307:179–186. doi: 10.1007/s00404-022-06494-4. [DOI] [PubMed] [Google Scholar]
- 15.Biscaldi E, Barra F, Leone Roberti Maggiore U, et al. Other imaging techniques: Double-contrast barium enema, endoscopic ultrasonography, multidetector CT enema, and computed tomography colonoscopy. Best Pract Res Clin Obstet Gynaecol. 2021;71:64–77. doi: 10.1016/j.bpobgyn.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Biscaldi E, Ferrero S, Fulcheri E, et al. Multislice CT enteroclysis in the diagnosis of bowel endometriosis. Eur Radiol. 2007;17:211–219. doi: 10.1007/s00330-006-0364-5. [DOI] [PubMed] [Google Scholar]
- 17.Burla L, Scheiner D, Hotker AM, et al. Structured manual for MRI assessment of deep infiltrating endometriosis using the ENZIAN classification. Arch Gynecol Obstet. 2021;303:751–757. doi: 10.1007/s00404-020-05892-w. [DOI] [PubMed] [Google Scholar]
- 18.Burla L, Scheiner D, Samartzis EP, et al. The ENZIAN score as a preoperative MRI-based classification instrument for deep infiltrating endometriosis. Arch Gynecol Obstet. 2019;300:109–116. doi: 10.1007/s00404-019-05157-1. [DOI] [PubMed] [Google Scholar]
- 19.Chaabane S, Nguyen Xuan HT, Paternostre A, et al. [Endometriosis: Assessment of the Ultrasound-Based Endometriosis Staging System score (UBESS) in predicting surgical difficulty]. Gynecol Obstet Fertil Senol. 2019;47:265–272. doi: 10.1016/j.gofs.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Di Giovanni A, Casarella L, Coppola M, et al. Ultrasound Evaluation of Retrocervical and Parametrial Deep Endometriosis on the Basis of Surgical Anatomic Landmarks. J Minim Invasive Gynecol. 2022;29:1140–1148. doi: 10.1016/j.jmig.2022.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Di Giovanni A, Montanari E, Hudelist G, et al. Comparison Between Sonography-Based and Surgical Evaluation of Endometriotic Lesions Using the #Enzian Classification - A Retrospective Data Analysis. Ultraschall Med. 2023;44:290–298. doi: 10.1055/a-1713-3573. [DOI] [PubMed] [Google Scholar]
- 22.Di Paola V, Manfredi R, Castelli F, et al. Detection and localization of deep endometriosis by means of MRI and correlation with the ENZIAN score. Eur J Radiol. 2015;84:568–574. doi: 10.1016/j.ejrad.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Enzelsberger SH, Oppelt P, Nirgianakis K, et al. Preoperative application of the Enzian classification for endometriosis (The cEnzian Study): A prospective international multicenter study. BJOG. 2022;129:2052–2061. doi: 10.1111/1471-0528.17235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espada M, Leonardi M, Aas-Eng K, et al. A Multicenter International Temporal and External Validation Study of the Ultrasound-based Endometriosis Staging System. J Minim Invasive Gynecol. 2021;28:57–562. doi: 10.1016/j.jmig.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Fendal Tunca A, Iliman DE, Akdogan Gemici A, et al. Predictive value of preoperative MRI using the #ENZIAN classification score in patients with deep infiltrating endometriosis. Arch Gynecol Obstet. 2023;307:215–220. doi: 10.1007/s00404-022-06451-1. [DOI] [PubMed] [Google Scholar]
- 26.Ferrero S, Biscaldi E, Morotti M, et al. Multidetector computerized tomography enteroclysis vs. rectal water contrast transvaginal ultrasonography in determining the presence and extent of bowel endometriosis. Ultrasound Obstet Gynecol. 2011;37:603–613. doi: 10.1002/uog.8971. [DOI] [PubMed] [Google Scholar]
- 27.Ferrero S, Biscaldi E, Vellone VG, et al. Computed tomographic colonography vs rectal water- contrast transvaginal sonography in diagnosis of rectosigmoid endometriosis: a pilot study. Ultrasound Obstet Gynecol. 2017;49:515–523. doi: 10.1002/uog.15905. [DOI] [PubMed] [Google Scholar]
- 28.Fratelli N, Scioscia M, Bassi E, et al. Transvaginal sonography for preoperative assessment of deep endometriosis. J Clin Ultrasound. 2013;41:69–75. doi: 10.1002/jcu.22018. [DOI] [PubMed] [Google Scholar]
- 29.Gerges B, Li W, Leonardi M, et al. Optimal imaging modality for detection of rectosigmoid deep endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021a;58:190–200. doi: 10.1002/uog.23148. [DOI] [PubMed] [Google Scholar]
- 30.Gerges B, Li W, Leonardi M, et al. Meta-analysis and systematic review to determine the optimal imaging modality for the detection of uterosacral ligaments/torus uterinus, rectovaginal septum and vaginal deep endometriosis. Hum Reprod Open. 2021b;2021:hoab041. doi: 10.1093/hropen/hoab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerges B, Li W, Leonardi M, et al. Meta-analysis and systematic review to determine the optimal imaging modality for the detection of bladder deep endometriosis. Eur J Obstet Gynecol Reprod Biol. 2021c;261:124–133. doi: 10.1016/j.ejogrb.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Goncalves MO, Siufi Neto J, Andres MP, et al. Systematic evaluation of endometriosis by transvaginal ultrasound can accurately replace diagnostic laparoscopy, mainly for deep and ovarian endometriosis. Hum Reprod. 2021;36:1492–1500. doi: 10.1093/humrep/deab085. [DOI] [PubMed] [Google Scholar]
- 33.Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46:534–545. doi: 10.1002/uog.15667. [DOI] [PubMed] [Google Scholar]
- 34.Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016a;48:318–332. doi: 10.1002/uog.15955. [DOI] [PubMed] [Google Scholar]
- 35.Guerriero S, Ajossa S, Orozco R, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in the rectosigmoid: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2016b;47:281–289. doi: 10.1002/uog.15662. [DOI] [PubMed] [Google Scholar]
- 36.Guerriero S, Saba L, Pascual MA, et al. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta- analysis. Ultrasound Obstet Gynecol. 2018a;51:586–595. doi: 10.1002/uog.18961. [DOI] [PubMed] [Google Scholar]
- 37.Guerriero S, Alcazar JL, Pascual MA, et al. Deep Infiltrating Endometriosis: Comparison Between 2-Dimensional Ultrasonography (US), 3-Dimensional US, and Magnetic Resonance Imaging. J Ultrasound Med. 2018b;37:1511–1521. doi: 10.1002/jum.14496. [DOI] [PubMed] [Google Scholar]
- 38.Guerriero S, Martinez L, Gomez I, et al. Diagnostic accuracy of transvaginal sonography for detecting parametrial involvement in women with deep endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;58:669–676. doi: 10.1002/uog.23754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendee WR, Edwards FM. ALARA and an integrated approach to radiation protection. Semin Nucl Med. 1986;16:142–150. doi: 10.1016/s0001-2998(86)80027-7. [DOI] [PubMed] [Google Scholar]
- 41.Holland TK, Yazbek J, Cutner A, et al. Value of transvaginal ultrasound in assessing severity of pelvic endometriosis. Ultrasound Obstet Gynecol. 2010;36:241–248. doi: 10.1002/uog.7689. [DOI] [PubMed] [Google Scholar]
- 42.Hottat N, Larrousse C, Anaf V, et al. Endometriosis: contribution of 3.0-T pelvic MR imaging in preoperative assessment-- initial results. Radiology. 2009;253:126–134. doi: 10.1148/radiol.2531082113. [DOI] [PubMed] [Google Scholar]
- 43.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Evidence Levels of Evidence (Introductory Document): Oxford Centre for Evidence-Based Medicine. [Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/levels-of-evidence-introductory-document. ]
- 44.Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257–263. doi: 10.1002/uog.8858. [DOI] [PubMed] [Google Scholar]
- 45.Hudelist G, Valentin L, Saridogan E, et al. What to choose and why to use - a critical review on the clinical relevance of rASRM, EFI and Enzian classifications of endometriosis. Facts Views Vis Obgyn. 2021a;13:331–338. doi: 10.52054/FVVO.13.4.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hudelist G, Montanari E, Salama M, et al. Comparison between Sonography-based and Surgical Extent of Deep Endometriosis Using the Enzian Classification - A Prospective Diagnostic Accuracy Study. J Minim Invasive Gynecol. 2021b;28:1643–1649.:e1. doi: 10.1016/j.jmig.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 47. International working group of AAGL, ESGE, ESHRE and WES, Vermeulen N, Abrao MS, et al. Endometriosis classification, staging and reporting systems: a review on the road to a universally accepted endometriosis classification. Facts Views Vis Obgyn. 2021;13:305–330. doi: 10.52054/FVVO.13.3.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jha P, Sakala M, Chamie LP, et al. Endometriosis MRI lexicon: consensus statement from the society of abdominal radiology endometriosis disease-focused panel. Abdom Radiol (NY) 2020;45:1552–1568. doi: 10.1007/s00261-019-02291-x. [DOI] [PubMed] [Google Scholar]
- 49.Keckstein J, Saridogan E, Ulrich UA, et al. The #Enzian classification: A comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand. 2021;100:1165–1175. doi: 10.1111/aogs.14099. [DOI] [PubMed] [Google Scholar]
- 50.Keckstein J, Ulrich U, Possover M, et al. ENZIAN-Klassifikation der tief infiltrierenden Endometriose. Zentralbl Gynäkol. 2003;125:291. doi: 10.1055/s-2005-836904. [DOI] [PubMed] [Google Scholar]
- 51.Leonardi M, Espada M, Choi S, et al. Transvaginal Ultrasound Can Accurately Predict the American Society of Reproductive Medicine Stage of Endometriosis Assigned at Laparoscopy. J Minim Invasive Gynecol. 2020;27:1581–7.:e1. doi: 10.1016/j.jmig.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Leonardi M, Uzuner C, Mestdagh W, et al. Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: prospective international pilot study. Ultrasound Obstet Gynecol. 2022;60:404–413. doi: 10.1002/uog.24936. [DOI] [PubMed] [Google Scholar]
- 53.Manganaro L, Celli V, Dolciami M, et al. Int J Environ Res Public Health. Can New ENZIAN Score 2020 Represent a Staging System Improving MRI Structured Report. 2021;18:22. doi: 10.3390/ijerph18199949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariani LL, Mancarella M, Novara L, et al. Sonographic features of endometriosis infiltrating the lateral parametrium. J Gynecol Obstet Hum Reprod. 2021;50:102–116. doi: 10.1016/j.jogoh.2021.102116. [DOI] [PubMed] [Google Scholar]
- 55.McAlister S, McGain F, Petersen M, et al. The carbon footprint of hospital diagnostic imaging in Australia. Lancet Reg Health West Pac. 2022;24:100459. doi: 10.1016/j.lanwpc.2022.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611–621. doi: 10.1007/s00404-014-3470-7. [DOI] [PubMed] [Google Scholar]
- 57.Menakaya U, Reid S, Lu C, et al. Performance of ultrasound-based endometriosis staging system (UBESS) for predicting level of complexity of laparoscopic surgery for endometriosis. Ultrasound Obstet Gynecol. 2016;48:786–795. doi: 10.1002/uog.15858. [DOI] [PubMed] [Google Scholar]
- 58.Montanari E, Bokor A, Szabo G, et al. Comparison of #Enzian classification and revised American Society for Reproductive Medicine stages for the description of disease extent in women with deep endometriosis. Hum Reprod. 2022a;37:2359–2365. doi: 10.1093/humrep/deac187. [DOI] [PubMed] [Google Scholar]
- 59.Montanari E, Bokor A, Szabo G, et al. Accuracy of sonography for non-invasive detection of ovarian and deep endometriosis using #Enzian classification: prospective multicenter diagnostic accuracy study. Ultrasound Obstet Gynecol. 2022b;59:385–391. doi: 10.1002/uog.24833. [DOI] [PubMed] [Google Scholar]
- 60.Moura APC, Ribeiro H, Bernardo WM, et al. Accuracy of transvaginal sonography versus magnetic resonance imaging in the diagnosis of rectosigmoid endometriosis: Systematic review and meta-analysis. PLoS ONE. 2019;14:e0214842. doi: 10.1371/journal.pone.0214842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591. doi: 10.1002/14651858.CD009591.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noventa M, Scioscia M, Schincariol M, et al. Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis. Diagnostics (Basel) 2019;9:17. doi: 10.3390/diagnostics9040225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pascoal E, Wessels JM, Aas-Eng MK, et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet Gynecol. 2022;60:309–327. doi: 10.1002/uog.24892. [DOI] [PubMed] [Google Scholar]
- 64.Pereira AMG, Brizon VSC, Carvas Junior N, et al. Can Enhanced Techniques Improve the Diagnostic Accuracy of Transvaginal Sonography and Magnetic Resonance Imaging for Rectosigmoid Endometriosis? A Systematic Review and Meta-analysis. J Obstet Gynaecol Can. 2020;42:488–499.:e4. doi: 10.1016/j.jogc.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 65.Piessens S, Healey M, Maher P, et al. Aust N Z J Obstet Gynaecol. Can anyone screen for deep infiltrating endometriosis with transvaginal ultrasound. 2014;54:462–468. doi: 10.1111/ajo.12242. [DOI] [PubMed] [Google Scholar]
- 66. American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: Fertil Steril. 1996;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 67.Roditis A, Florin M, Rousset P, et al. Accuracy of combined physical examination, transvaginal ultrasonography, and magnetic resonance imaging to diagnose deep endometriosis. Fertil Steril. 2023;119:634–643. doi: 10.1016/j.fertnstert.2022.12.025. [DOI] [PubMed] [Google Scholar]
- 68.Rousset P, Florin M, Bharwani N, et al. Deep pelvic infiltrating endometriosis: MRI consensus lexicon and compartment-based approach from the ENDOVALIRM group. Diagn Interv Imaging. 2023;104:95–112. doi: 10.1016/j.diii.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Saccardi C, Vitagliano A, Litta P. Bladder endometriosis: a summary of current evidence. Minerva Ginecol. 2017;69:468–476. doi: 10.23736/S0026-4784.17.04059-X. [DOI] [PubMed] [Google Scholar]
- 70.Scioscia M, Scardapane A, Virgilio BA, et al. Ultrasound of the Uterosacral Ligament, Parametrium, and Paracervix: Disagreement in Terminology between Imaging Anatomy and Modern Gynecologic Surgery. J Clin Med. 2021;10:437. doi: 10.3390/jcm10030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stabile Ianora AA, Moschetta M, Lorusso F, et al. Rectosigmoid endometriosis: comparison between CT water enema and video laparoscopy. Clin Radiol. 2013;68:895–901. doi: 10.1016/j.crad.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Szabo G, Hudelist G, Madar I, et al. Diagnostic accuracy of the IDEA protocol for non invasive diagnosis of rectosigmoid DE - a prospective cohort study. Ultraschall Med. 2024;45:61–68. doi: 10.1055/a-2034-2022. [DOI] [PubMed] [Google Scholar]
- 73.Thomassin-Naggara I, Lamrabet S, Crestani A, et al. Magnetic resonance imaging classification of deep pelvic endometriosis: description and impact on surgical management. Hum Reprod. 2020;35:1589–1600. doi: 10.1093/humrep/deaa103. [DOI] [PubMed] [Google Scholar]
- 74.Thomassin-Naggara I, Monroc M, Chauveau B, et al. Multicenter External Validation of the Deep Pelvic Endometriosis Index Magnetic Resonance Imaging Score. JAMA Netw Open. 2023;6:e2311686. doi: 10.1001/jamanetworkopen.2023.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timmerman D, Planchamp F, Bourne T, et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on preoperative diagnosis of ovarian tumors. Ultrasound Obstet Gynecol. 2021;58:148–168. doi: 10.1002/uog.23635. [DOI] [PubMed] [Google Scholar]
- 76.Tomassetti C, Bafort C, Vanhie A, et al. Estimation of the Endometriosis Fertility Index prior to operative laparoscopy. Hum Reprod. 2021;36:636–646. doi: 10.1093/humrep/deaa346. [DOI] [PubMed] [Google Scholar]
- 77.Vesali S, Razavi M, Rezaeinejad M, et al. Endometriosis fertility index for predicting non-assisted reproductive technology pregnancy after endometriosis surgery: a systematic review and meta-analysis. BJOG. 2020;127:800–809. doi: 10.1111/1471-0528.16107. [DOI] [PubMed] [Google Scholar]
- 78.Widschwendter P, Kohler M, Friedl T, et al. Diagnosis of presence and extent of deep infiltrating endometriosis by preoperative MRI-improvement of staging accuracy by expert training. J Gynecol Obstet Hum Reprod. 2022;51:102236. doi: 10.1016/j.jogoh.2021.102236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Revised American Society for Reproductive Medicine (rASRM) classification of endometriosis. Reprinted from the Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21. Copyright© 1997 American Society for Reproductive Medicine, with permission from Elsevier. All rights reserved.
#Enzian classification system for women with superficial, ovarian and deep endometriosis. Reprinted from Keckstein et al. (2021), with permission from J. Keckstein. Copyright© 2021 The Authors. Published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG). Sacrouterine ligg/USL, uterosacral ligaments.
Ultrasound-based Endometriosis Staging System (UBESS), with sonographic features demonstrable on transvaginal ultrasound (TVS) and its prediction of level of surgical complexity. Adapted from Menakaya et al. (2016), with permission from ISUOG. SVG, sonovaginography.
Endometriosis fertility index (EFI) system. This score predicts fertility outcome for women who attempt non-in-vitro fertilization conception following surgically documented endometriosis. Reprinted from Adamson and Pasta, 2010. Copyright© 2010 American Society for Reproductive Medicine, with permission from Elsevier. All rights reserved. AFS, American Fertility Society.
Magnetic resonance imaging (MRI) lexicon and deep pelvic endometriosis index (dPEI) classification: low extension (score 1 or 2), moderate extension (score 3 or 4) or severe extension (score 5 or more). Reproduced from Rousset et al. (2023). Copyright© 2022 The Author(s). Published by Elsevier Masson SAS on behalf of Société française de radiologie. All rights reserved.


