Abstract
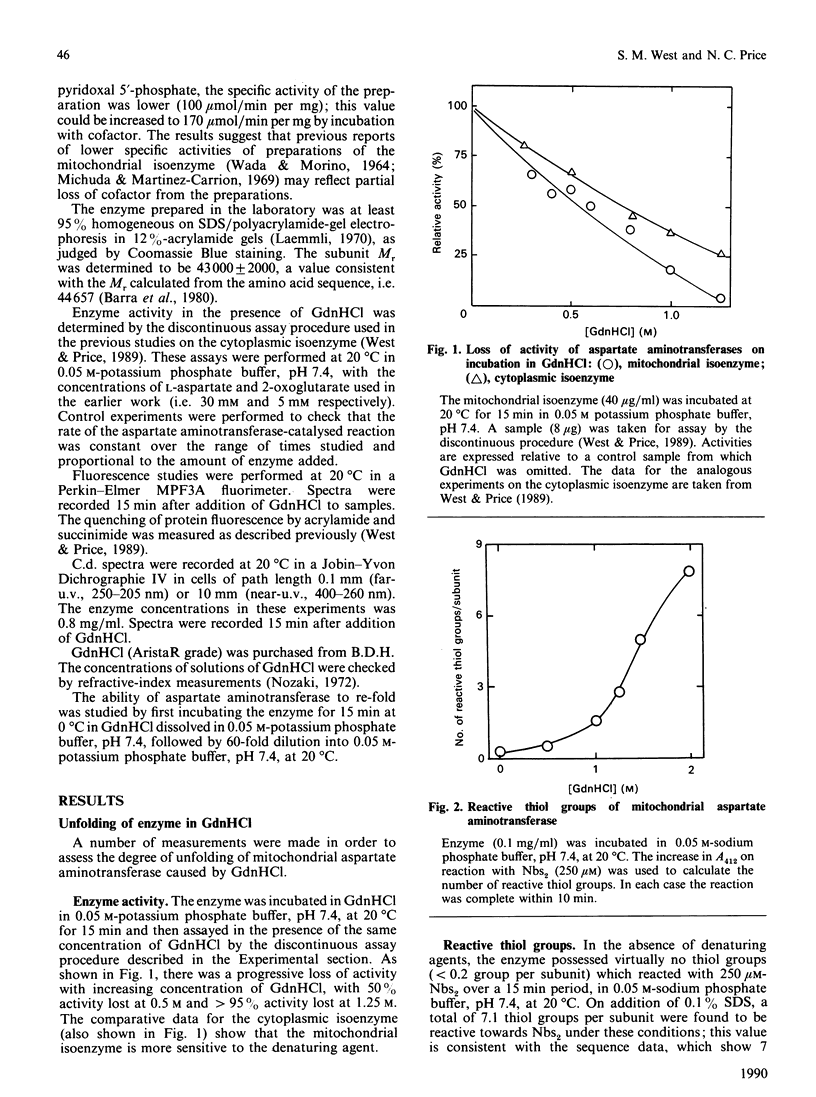
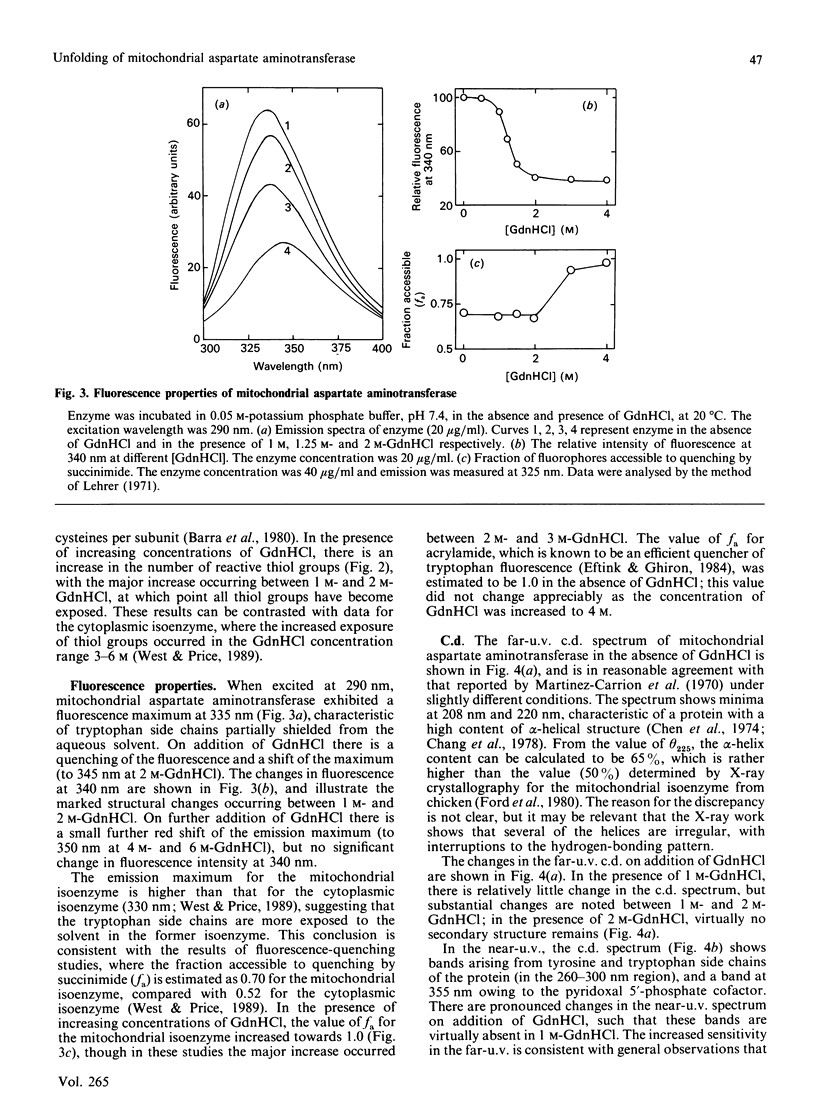
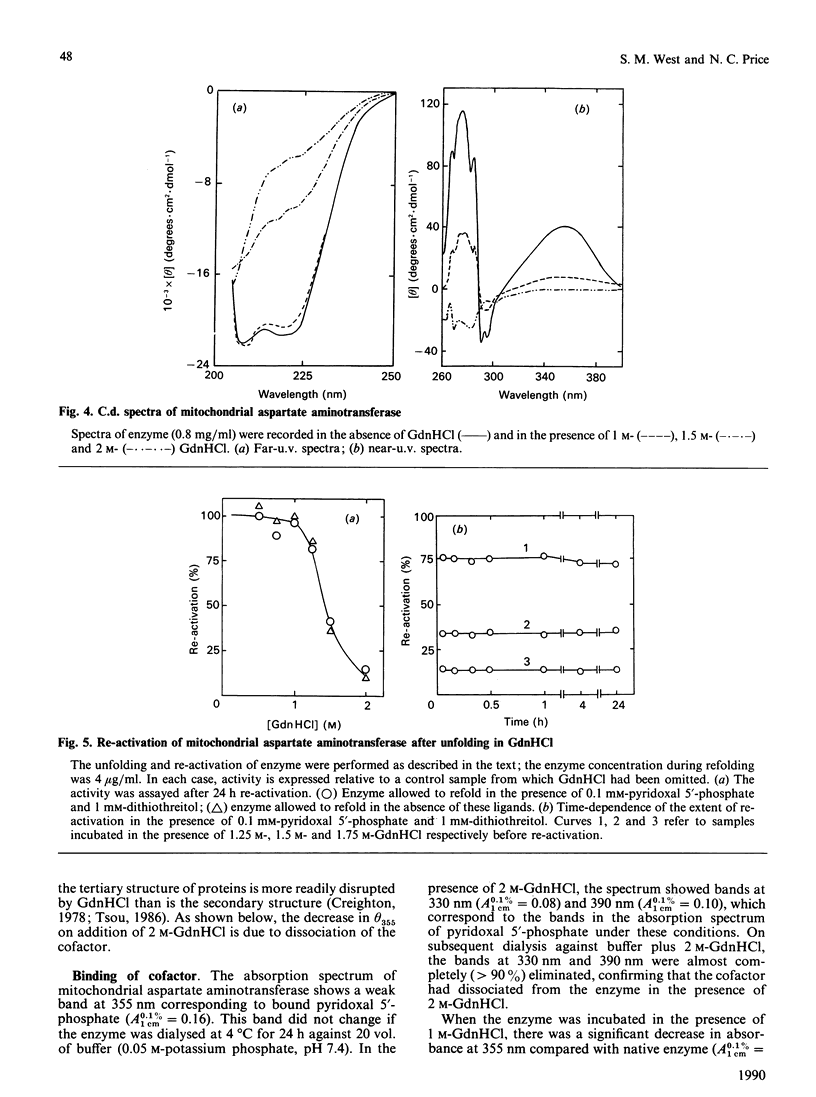
The unfolding of the mitochondrial isoenzyme of aspartate aminotransferase from pig heart in solutions of guanidinium chloride (GdnHCl) has been studied. By a number of criteria (enzyme activity, protein fluorescence, c.d., thiol-group reactivity), the enzyme was judged to be almost completely unfolded in 2 M-GdnHCl. On dilution of the GdnHCl, no re-activation of the enzyme could be observed, whether or not pyridoxal 5'-phosphate and dithiothreitol were present. The behaviour of the mitochondrial isoenzyme is in marked contrast with that of the cytoplasmic isoenzyme [West & Price (1989) Biochem. J. 261, 189-196], despite the similarities in the amino acid sequences and tertiary structures of the two isoenzymes. The implications of these findings for the process of folding and assembly of the mitochondrial isoenzyme in vivo are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri F., Mattingly J. R., Jr, Rodriguez-Berrocal F. J., Youssef J., Iriarte A., Wu T. H., Martinez-Carrion M. Isolation and properties of a liver mitochondrial precursor protein to aspartate aminotransferase expressed in Escherichia coli. J Biol Chem. 1989 Mar 25;264(9):4782–4786. [PubMed] [Google Scholar]
- Barra D., Bossa F., Doonan S., Fahmy H. M., Hughes G. J., Martini F., Petruzzelli R., Wittmann-Liebold B. The cytosolic and mitochondrial aspartate aminotransferases from pig heart. A comparison of their primary structures, predicted secondary structures and some physical properties. Eur J Biochem. 1980 Jul;108(2):405–414. doi: 10.1111/j.1432-1033.1980.tb04736.x. [DOI] [PubMed] [Google Scholar]
- Barra D., Bossa F., Doonan S., Fahmy H. M., Martini F., Hughes G. J. Large-scale purification and some properties of the mitochondrial aspartate aminotransferase from pig heart. Eur J Biochem. 1976 May 1;64(2):519–526. doi: 10.1111/j.1432-1033.1976.tb10331.x. [DOI] [PubMed] [Google Scholar]
- Bychkova V. E., Pain R. H., Ptitsyn O. B. The 'molten globule' state is involved in the translocation of proteins across membranes? FEBS Lett. 1988 Oct 10;238(2):231–234. doi: 10.1016/0014-5793(88)80485-x. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Eichele G., Jansonius J. N. Three-dimensional structure of a pyridoxal-phosphate-dependent enzyme, mitochondrial aspartate aminotransferase. Proc Natl Acad Sci U S A. 1980 May;77(5):2559–2563. doi: 10.1073/pnas.77.5.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Jaussi R., Behra R., Giannattasio S., Flura T., Christen P. Expression of cDNAs encoding the precursor and the mature form of chicken mitochondrial aspartate aminotransferase in Escherichia coli. J Biol Chem. 1987 Sep 15;262(26):12434–12437. [PubMed] [Google Scholar]
- Jaussi R., Cotton B., Juretić N., Christen P., Schümperli D. The primary structure of the precursor of chicken mitochondrial aspartate aminotransferase. Cloning and sequence analysis of cDNA. J Biol Chem. 1985 Dec 25;260(30):16060–16063. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Tiemeier D. C., Peterson D. L. Conformational properties of the isoenzymes of aspartate transaminase and the enzyme-substrate complexes. Biochemistry. 1970 Jun 23;9(13):2574–2582. doi: 10.1021/bi00815a004. [DOI] [PubMed] [Google Scholar]
- Meyer D. I. Preprotein conformation: the year's major theme in translocation studies. Trends Biochem Sci. 1988 Dec;13(12):471–474. doi: 10.1016/0968-0004(88)90233-2. [DOI] [PubMed] [Google Scholar]
- Michuda C. M., Martinez-Carrion M. Mitochondrial aspartate transaminase. II. Isolation and characterization of the multiple forms. Biochemistry. 1969 Mar;8(3):1095–1105. doi: 10.1021/bi00831a041. [DOI] [PubMed] [Google Scholar]
- Nishi T., Nagashima F., Tanase S., Fukumoto Y., Joh T., Shimada K., Matsukado Y., Ushio Y., Morino Y. Import and processing of precursor to mitochondrial aspartate aminotransferase. Structure-function relationships of the presequence. J Biol Chem. 1989 Apr 15;264(11):6044–6051. [PubMed] [Google Scholar]
- Nozaki Y. The preparation of guanidine hydrochloride. Methods Enzymol. 1972;26:43–50. doi: 10.1016/s0076-6879(72)26005-0. [DOI] [PubMed] [Google Scholar]
- WADA H., MORINO Y. COMPARATIVE STUDIES ON GLUTAMIC-OXALACETIC TRANSAMINASES FROM THE MITOCHONDRIAL AND SOLUBLE FRACTIONS OF MAMMALIAN TISSUES. Vitam Horm. 1964;22:411–444. doi: 10.1016/s0083-6729(08)60346-5. [DOI] [PubMed] [Google Scholar]
- West S. M., Price N. C. The unfolding and refolding of cytoplasmic aspartate aminotransferase from pig heart. Biochem J. 1989 Jul 1;261(1):189–196. doi: 10.1042/bj2610189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. M., Price N. C. The unfolding and refolding of glutamate dehydrogenases from bovine liver, baker's yeast and Clostridium symbosium. Biochem J. 1988 Apr 1;251(1):135–139. doi: 10.1042/bj2510135. [DOI] [PMC free article] [PubMed] [Google Scholar]


