Abstract
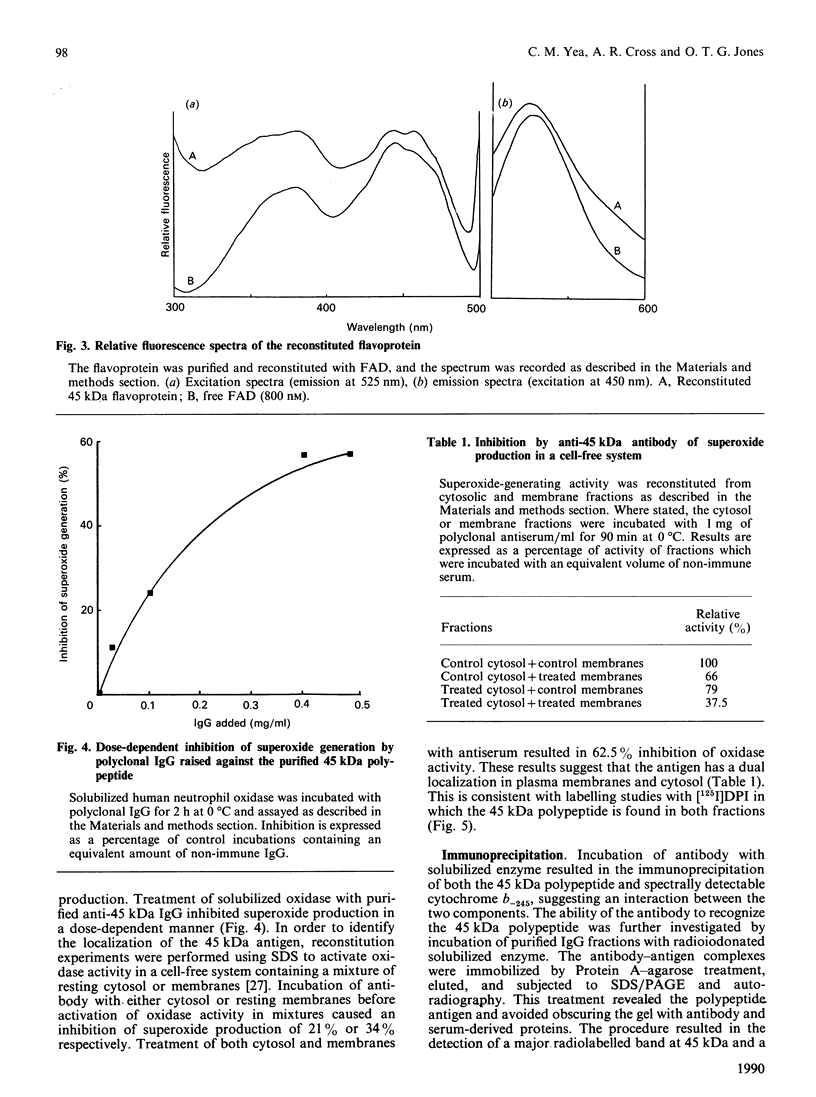
The 45 kDa diphenylene iodonium-binding flavoprotein of the human neutrophil superoxide-generating oxidase has been purified by affinity chromatography. The polypeptide was eluted from Blue Memsep or 2',5'-ADP-agarose columns with either NADP or low concentrations of the specific inhibitor diphenylene iodonium. The purified protein was shown to bind FAD at a ratio of 1.09 mol of FAD/mol of protein. The reconstituted flavoprotein had a fluorescence spectrum similar, but not identical, to that of free FAD. It had an isoelectric point of approx. 4.0. The reconstituted flavoprotein displayed no diaphorase activity towards a range of artificial electron acceptors. Polyclonal antibodies raised against the pure protein inhibited superoxide generation by solubilized oxidase in a dose-dependent manner, and inhibited superoxide generation when incubated with either cytosol or membrane fractions in a reconstituted system. These antibodies precipitated the 45 kDa polypeptide together with a haem-containing 23 kDa protein thought to be the small subunit of cytochrome b-245. Antibodies raised against cytochrome P-450 reductase also precipitated these two polypeptides. These results are consistent with the 45 kDa polypeptide being the flavoprotein of the neutrophil superoxide-generating oxidase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew P. W., Robertson A. K., Lowrie D. B., Cross A. R., Jones O. T. Induction of synthesis of components of the hydrogen peroxide-generating oxidase during activation of the human monocytic cell line U937 by interferon-gamma. Biochem J. 1987 Nov 15;248(1):281–283. doi: 10.1042/bj2480281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavite P. The superoxide-forming enzymatic system of phagocytes. Free Radic Biol Med. 1988;4(4):225–261. doi: 10.1016/0891-5849(88)90044-5. [DOI] [PubMed] [Google Scholar]
- Bellavite P., della Bianca V., Serra M. C., Papini E., Rossi F. NADPH oxidase of neutrophils forms superoxide anion but does not reduce cytochrome c and dichlorophenolindophenol. FEBS Lett. 1984 May 7;170(1):157–161. doi: 10.1016/0014-5793(84)81390-3. [DOI] [PubMed] [Google Scholar]
- Berton G., Dusi S., Serra M. C., Bellavite P., Rossi F. Studies on the NADPH oxidase of phagocytes. Production of a monoclonal antibody which blocks the enzymatic activity of pig neutrophil NADPH oxidase. J Biol Chem. 1989 Apr 5;264(10):5564–5568. [PubMed] [Google Scholar]
- Bolscher B. G., van Zwieten R., Kramer I. M., Weening R. S., Verhoeven A. J., Roos D. A phosphoprotein of Mr 47,000, defective in autosomal chronic granulomatous disease, copurifies with one of two soluble components required for NADPH:O2 oxidoreductase activity in human neutrophils. J Clin Invest. 1989 Mar;83(3):757–763. doi: 10.1172/JCI113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. Mechanism of the superoxide-producing oxidase of neutrophils. O2 is necessary for the fast reduction of cytochrome b-245 by NADPH. Biochem J. 1985 Mar 15;226(3):881–884. doi: 10.1042/bj2260881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984 Oct 15;223(2):337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R. The inhibitory effects of some iodonium compounds on the superoxide generating system of neutrophils and their failure to inhibit diaphorase activity. Biochem Pharmacol. 1987 Feb 15;36(4):489–493. doi: 10.1016/0006-2952(87)90356-x. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Babior B. M. Activation of the respiratory burst oxidase in a fully soluble system from human neutrophils. J Biol Chem. 1987 May 15;262(14):6450–6452. [PubMed] [Google Scholar]
- Curnutte J. T., Scott P. J., Mayo L. A. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1989 Feb;86(3):825–829. doi: 10.1073/pnas.86.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussiere J., Vignais P. V. Purification and properties of an O2-.-generating oxidase from bovine polymorphonuclear neutrophils. Biochemistry. 1985 Dec 3;24(25):7231–7239. doi: 10.1021/bi00346a032. [DOI] [PubMed] [Google Scholar]
- Doussière J., Vignais P. V. Immunological properties of O2.- generating oxidase from bovine neutrophils. FEBS Lett. 1988 Jul 18;234(2):362–366. doi: 10.1016/0014-5793(88)80117-0. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., Cross A. R., Jones O. T. Studies on the electron-transfer mechanism of the human neutrophil NADPH oxidase. Biochem J. 1989 Sep 1;262(2):575–579. doi: 10.1042/bj2620575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R. W., Hildebrandt A. G., Baron J., Netter K. J., Leibman K. A new spectral intermediate associated with cytochrome P-450 function in liver microsomes. Biochem Biophys Res Commun. 1971 Jan 8;42(1):132–139. doi: 10.1016/0006-291x(71)90372-x. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Glass G. A., DeLisle D. M., DeTogni P., Gabig T. G., Magee B. H., Markert M., Babior B. M. The respiratory burst oxidase of human neutrophils. Further studies of the purified enzyme. J Biol Chem. 1986 Oct 5;261(28):13247–13251. [PubMed] [Google Scholar]
- Green T. R., Pratt K. L. Purification of the solubilized NADPH:O2 oxidoreductase of human neutrophils. Isolation of its catalytically inactive cytochrome b and flavoprotein redox centers. J Biol Chem. 1988 Apr 25;263(12):5617–5623. [PubMed] [Google Scholar]
- Hancock J. T., Jones O. T. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987 Feb 15;242(1):103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B., Jones O. T. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987 Sep 1;246(2):325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maly F. E., Cross A. R., Jones O. T., Wolf-Vorbeck G., Walker C., Dahinden C. A., De Weck A. L. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988 Apr 1;140(7):2334–2339. [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Parkinson J. F., Gabig T. G. Isolation of the respiratory burst oxidase: the role of a flavoprotein component. J Bioenerg Biomembr. 1988 Dec;20(6):653–677. doi: 10.1007/BF00762547. [DOI] [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I., Bloxham D. P. Specific labelling of a constituent polypeptide of bovine heart mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone reductase by the inhibitor diphenyleneiodonium. Biochem J. 1977 Jun 1;163(3):605–615. doi: 10.1042/bj1630605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane F., Takahashi K., Koyama J. Purification and characterization of a membrane-bound NADPH-cytochrome c reductase capable of catalyzing menadione-dependent O2- formation in guinea pig polymorphonuclear leukocytes. J Biochem. 1984 Sep;96(3):671–678. doi: 10.1093/oxfordjournals.jbchem.a134884. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Curnutte J. T., Babior B. M. Affinity labeling of the cytosolic and membrane components of the respiratory burst oxidase by the 2',3'-dialdehyde derivative of NADPH. Evidence for a cytosolic location of the nucleotide-binding site in the resting cell. J Biol Chem. 1989 Feb 5;264(4):1958–1962. [PubMed] [Google Scholar]
- Takasugi S., Ishida K., Takeshige K., Minakami S. Effect of 2',3'-dialdehyde NADPH on activation of superoxide-producing NADPH oxidase in a cell-free system of pig neutrophils. J Biochem. 1989 Feb;105(2):155–157. doi: 10.1093/oxfordjournals.jbchem.a122630. [DOI] [PubMed] [Google Scholar]
- Takayama H., Iwaki S., Tamoto K., Koyama J. Inhibition of the O2- -generating NADPH oxidase of guinea-pig polymorphonuclear leukocytes by rabbit antibody to homologous liver NADPH-cytochrome c (P-450) reductase. Biochim Biophys Acta. 1984 Jun 15;799(2):151–157. doi: 10.1016/0304-4165(84)90289-7. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]




