Abstract
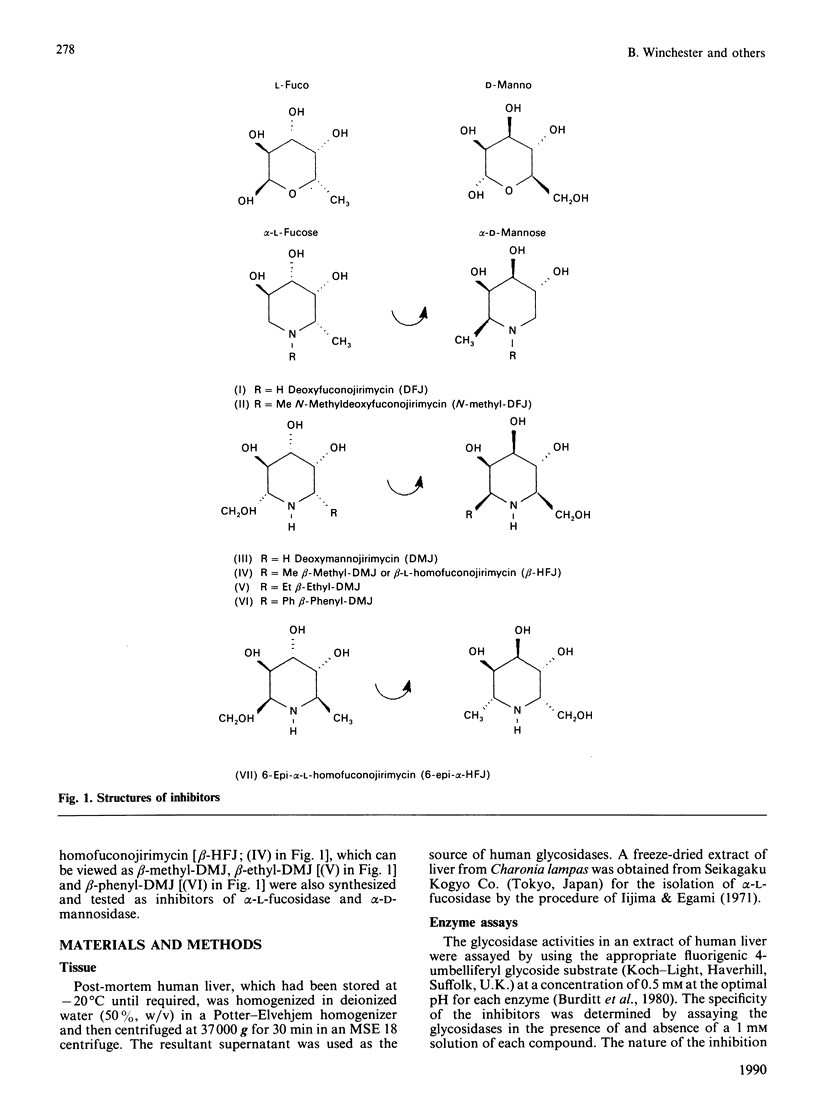
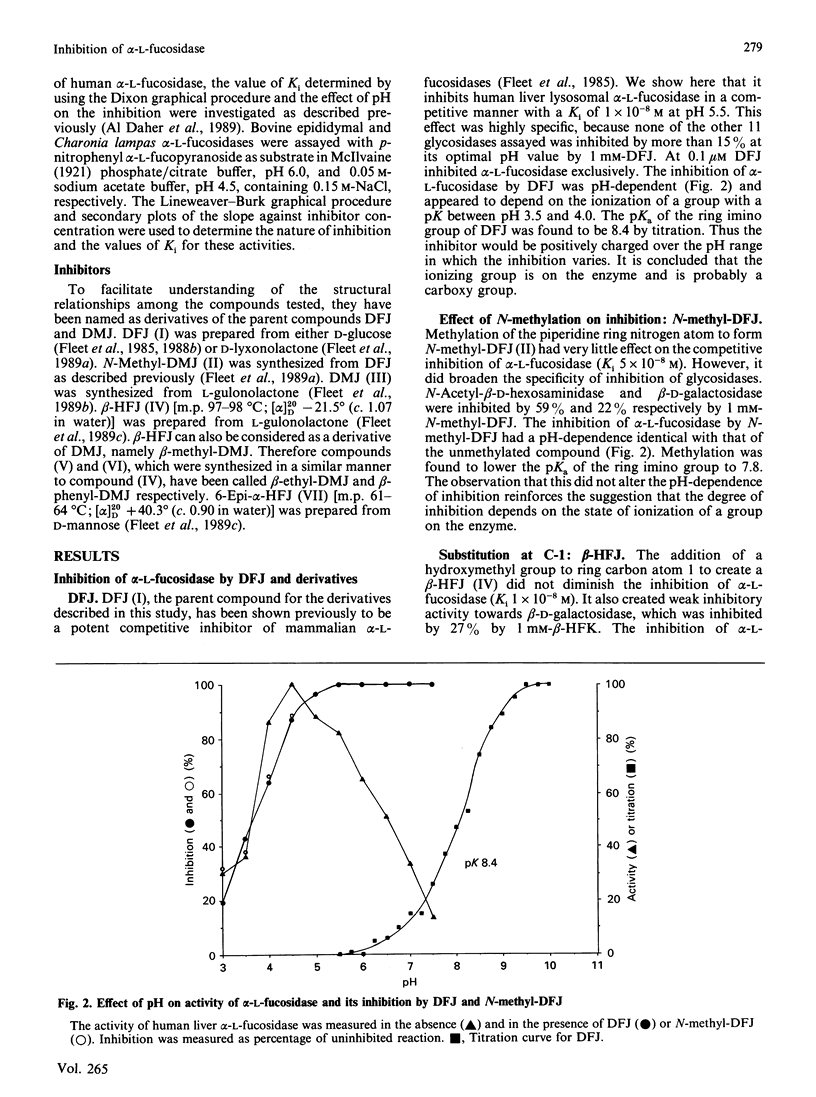
Deoxyfuconojirimycin (1,5-dideoxy-1,5-imino-L-fucitol) is a potent, specific and competitive inhibitor (Ki 1 x 10(-8) M) of human liver alpha-L-fucosidase (EC 3.2.1.51). Six structural analogues of this compound were synthesized and tested for their ability to inhibit alpha-L-fucosidase and other human liver glycosidases. It is concluded that the minimum structural requirement for inhibition of alpha-L-fucosidase is the correct configuration of the hydroxy groups at the piperidine ring carbon atoms 2, 3 and 4. Different substituents in either configuration at carbon atom 1 (i.e. 1 alpha- and beta-homofuconojirimycins) and at carbon atom 5 may alter the potency but do not destroy the inhibition of alpha-L-fucosidase. The pH-dependency of the inhibition by these amino sugars suggests very strongly that inhibition results from the formation of an ion-pair between the protonated inhibitor and a carboxylate group in the active site of the enzyme. Deoxymannojirimycin (1,5-dideoxy-1,5-imino-D-mannitol) is also a more potent inhibitor of alpha-L-fucosidase than of alpha-D-mannosidase. This can be explained by viewing deoxymannojirimycin as beta-L-homofuconojirimycin lacking the 5-methyl group. Conversely, beta-L-homo analogues of fuconojirimycin can also be regarded as derivatives of deoxymannojirimycin. This has permitted deductions to be made about the structural requirements of inhibitors of alpha- and beta-D-mannosidases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burditt L. J., Chotai K., Hirani S., Nugent P. G., Winchester B. G., Blakemore W. F. Biochemical studies on a case of feline mannosidosis. Biochem J. 1980 Sep 1;189(3):467–473. doi: 10.1042/bj1890467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci di Bello I., Dorling P., Winchester B. The storage products in genetic and swainsonine-induced human mannosidosis. Biochem J. 1983 Dec 1;215(3):693–696. doi: 10.1042/bj2150693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci di Bello I., Fleet G., Namgoong S. K., Tadano K., Winchester B. Structure-activity relationship of swainsonine. Inhibition of human alpha-mannosidases by swainsonine analogues. Biochem J. 1989 May 1;259(3):855–861. doi: 10.1042/bj2590855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S. F., Dawson G. Purification and properties of two forms of human alpha-L-fucosidase. Biochim Biophys Acta. 1980 Aug 7;614(2):476–488. doi: 10.1016/0005-2744(80)90237-5. [DOI] [PubMed] [Google Scholar]
- Dennis J. W. Effects of swainsonine and polyinosinic:polycytidylic acid on murine tumor cell growth and metastasis. Cancer Res. 1986 Oct;46(10):5131–5136. [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fleet G. W., Karpas A., Dwek R. A., Fellows L. E., Tyms A. S., Petursson S., Namgoong S. K., Ramsden N. G., Smith P. W., Son J. C. Inhibition of HIV replication by amino-sugar derivatives. FEBS Lett. 1988 Sep 12;237(1-2):128–132. doi: 10.1016/0014-5793(88)80185-6. [DOI] [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Matsumoto K., White S. L., Olden K. Oligosaccharide modification by swainsonine treatment inhibits pulmonary colonization by B16-F10 murine melanoma cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1752–1756. doi: 10.1073/pnas.83.6.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima Y., Egami F. Purification of alpha-L-fucosidose from the liver of a marine gastropod, Chariona lampas. J Biochem. 1971 Jul;70(1):75–78. doi: 10.1093/oxfordjournals.jbchem.a129628. [DOI] [PubMed] [Google Scholar]
- Nishigaki M., Muramatsu T., Kobata A., Maeyama K. The broad aglycon specificity of alpha-l-fucosidase from marine gastropods. J Biochem. 1974 Mar;75(3):509–517. doi: 10.1093/oxfordjournals.jbchem.a130419. [DOI] [PubMed] [Google Scholar]
- Thorpe R., Robinson D. Purification and serological studies of human alpha-L-fucosidase in the normal and fucosidosis states. Clin Chim Acta. 1978 May 16;86(1):21–30. doi: 10.1016/0009-8981(78)90453-9. [DOI] [PubMed] [Google Scholar]
- Tyms A. S., Berrie E. M., Ryder T. A., Nash R. J., Hegarty M. P., Taylor D. L., Mobberley M. A., Davis J. M., Bell E. A., Jeffries D. J. Castanospermine and other plant alkaloid inhibitors of glucosidase activity block the growth of HIV. Lancet. 1987 Oct 31;2(8566):1025–1026. doi: 10.1016/s0140-6736(87)92588-8. [DOI] [PubMed] [Google Scholar]
- White W. J., Jr, Schray K. J., Alhadeff J. A. Studies on the catalytic residues at the active site of human liver alpha-L-fucosidase. Biochim Biophys Acta. 1985 Jul 1;829(3):303–310. doi: 10.1016/0167-4838(85)90237-7. [DOI] [PubMed] [Google Scholar]
- White W. J., Jr, Schray K. J., Legler G., Alhadeff J. A. Active-site-directed inactivation of human liver alpha-L-fucosidase by conduritol C trans-epoxide. Biochim Biophys Acta. 1986 Sep 26;873(2):198–203. doi: 10.1016/0167-4838(86)90046-4. [DOI] [PubMed] [Google Scholar]
- White W. J., Jr, Schray K. J., Legler G., Alhadeff J. A. Further studies on the catalytic mechanism of human liver alpha-L-fucosidase. Biochim Biophys Acta. 1987 Mar 18;912(1):132–138. doi: 10.1016/0167-4838(87)90256-1. [DOI] [PubMed] [Google Scholar]
- Wiederschain G. Y., Rosenfeld E. L. Specificity of pig kidney alpha-L-fucosidase and its action on different fragments of blood group A+H substance. Bull Soc Chim Biol (Paris) 1969 Oct 23;51(6):1075–1084. [PubMed] [Google Scholar]
- al Daher S., Fleet G., Namgoong S. K., Winchester B. Change in specificity of glycosidase inhibition by N-alkylation of amino sugars. Biochem J. 1989 Mar 1;258(2):613–615. doi: 10.1042/bj2580613. [DOI] [PMC free article] [PubMed] [Google Scholar]


