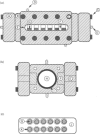
Abstract
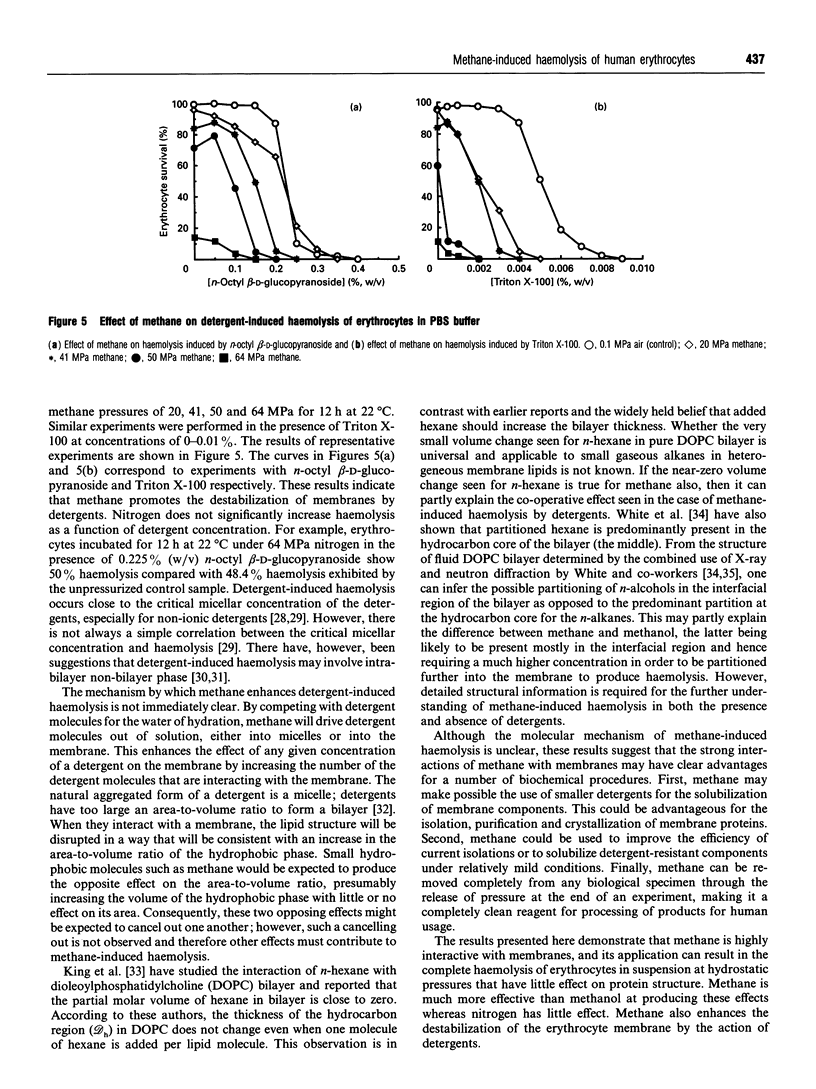
Human erythrocytes were exposed to high concentrations of methane and nitrogen through the application of elevated partial pressures of these gas molecules. Cell leakage (haemolysis) was measured for cells exposed to these gases under a wide range of experimental conditions. Application of methane produces haemolysis at pressures far below the hydrostatic pressures known to disrupt membrane or protein structure. The effects of changes in buffer, temperature, diffusion rate and detergents were studied. Methane acts co-operatively with detergents to produce haemolysis at much lower detergent concentration than is required in the absence of methane or in the presence of nitrogen. At sufficiently high concentrations of methane, all cells are haemolysed. Increased temperature enhances the effect. Methane produces 50% haemolysis at a concentration of about 0.33 M compared with about 7.5 M methanol required for the same degree of haemolysis.
Full text
PDF
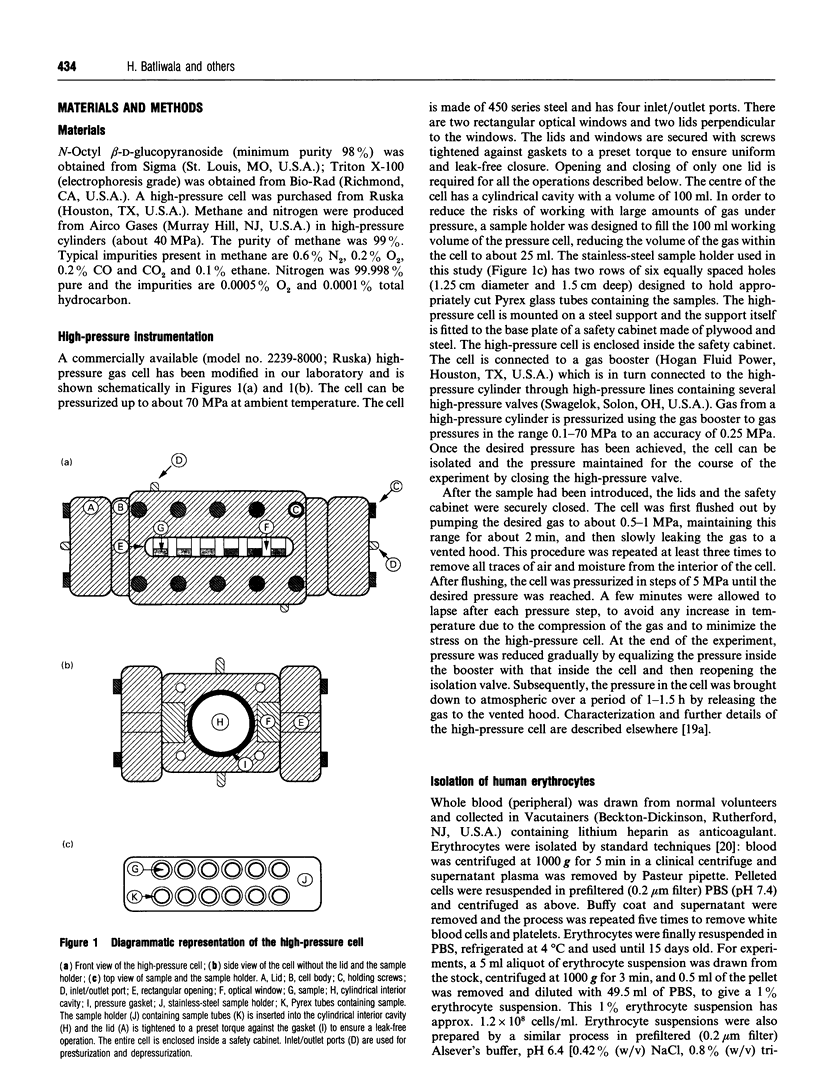
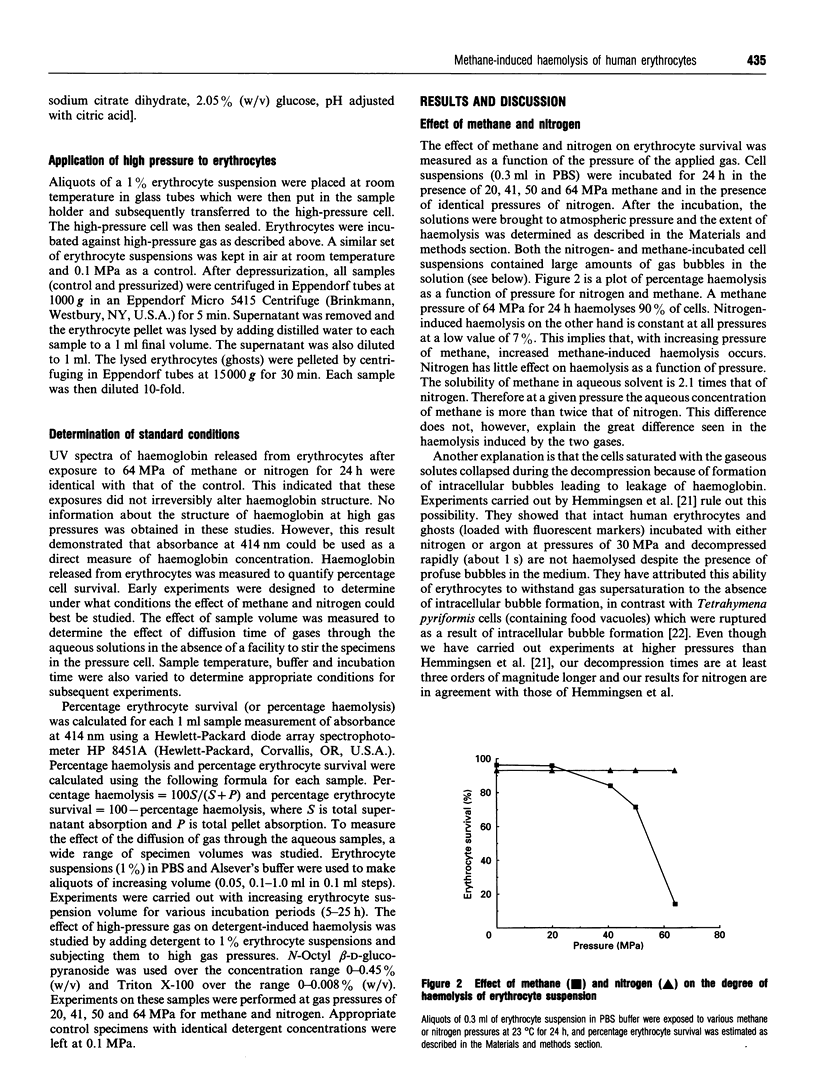
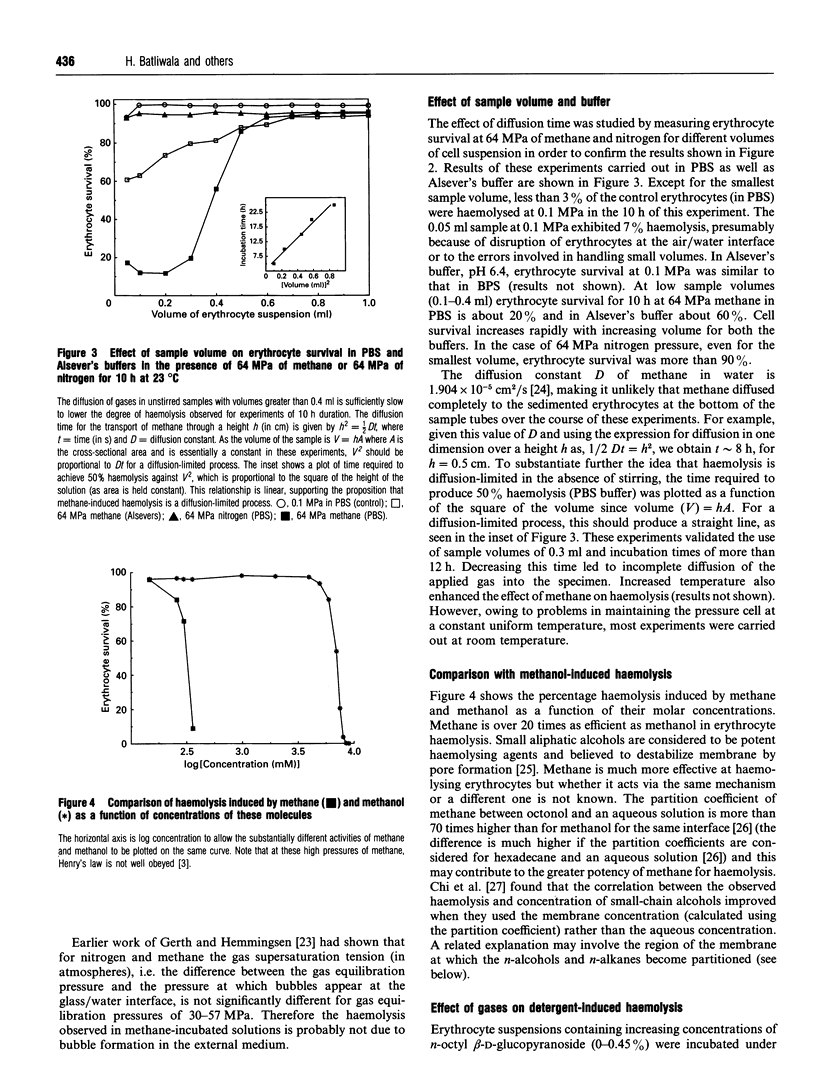

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braganza L. F., Worcester D. L. Hydrostatic pressure induces hydrocarbon chain interdigitation in single-component phospholipid bilayers. Biochemistry. 1986 May 6;25(9):2591–2596. doi: 10.1021/bi00357a047. [DOI] [PubMed] [Google Scholar]
- Chi L. M., Wu W. G. Mechanism of hemolysis of red blood cell mediated by ethanol. Biochim Biophys Acta. 1991 Feb 11;1062(1):46–50. doi: 10.1016/0005-2736(91)90333-4. [DOI] [PubMed] [Google Scholar]
- Chi L. M., Wu W. G., Sung K. L., Chien S. Biophysical correlates of lysophosphatidylcholine- and ethanol-mediated shape transformation and hemolysis of human erythrocytes. Membrane viscoelasticity and NMR measurement. Biochim Biophys Acta. 1990 Aug 24;1027(2):163–171. doi: 10.1016/0005-2736(90)90080-8. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Deckmann M., Haimovitz R., Shinitzky M. Selective release of integral proteins from human erythrocyte membranes by hydrostatic pressure. Biochim Biophys Acta. 1985 Dec 5;821(2):334–340. doi: 10.1016/0005-2736(85)90103-8. [DOI] [PubMed] [Google Scholar]
- Dodson B. A., Furmaniuk Z. W., Jr, Miller K. W. The physiological effects of hydrostatic pressure are not equivalent to those of helium pressure on Rana pipiens. J Physiol. 1985 May;362:233–244. doi: 10.1113/jphysiol.1985.sp015673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers A. S., Berkowitz B. A., d'Avignon D. A. Correlation between the anaesthetic effect of halothane and saturable binding in brain. Nature. 1987 Jul 9;328(6126):157–160. doi: 10.1038/328157a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994 Feb 17;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C., Klein R. A. Pressure and temperature effects on human red cell cation transport. J Membr Biol. 1982;68(1):47–56. doi: 10.1007/BF01872253. [DOI] [PubMed] [Google Scholar]
- Halle D., Yedgar S. Mild pressure induces resistance of erythrocytes to hemolysis by snake venom phospholipase A2. Biophys J. 1988 Sep;54(3):393–396. doi: 10.1016/S0006-3495(88)82972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen B. B., Steinberg N. A., Hemmingsen E. A. Intracellular gas supersaturation tolerances of erythrocytes and resealed ghosts. Biophys J. 1985 Apr;47(4):491–496. doi: 10.1016/S0006-3495(85)83942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans K. High pressure effects on proteins and other biomolecules. Annu Rev Biophys Bioeng. 1982;11:1–21. doi: 10.1146/annurev.bb.11.060182.000245. [DOI] [PubMed] [Google Scholar]
- Isomaa B., Hägerstrand H., Paatero G., Engblom A. C. Permeability alterations and antihaemolysis induced by amphiphiles in human erythrocytes. Biochim Biophys Acta. 1986 Sep 11;860(3):510–524. doi: 10.1016/0005-2736(86)90548-1. [DOI] [PubMed] [Google Scholar]
- Isomaa B., Hägerstrand H., Paatero G. Shape transformations induced by amphiphiles in erythrocytes. Biochim Biophys Acta. 1987 May 12;899(1):93–103. doi: 10.1016/0005-2736(87)90243-4. [DOI] [PubMed] [Google Scholar]
- King G. I., Jacobs R. E., White S. H. Hexane dissolved in dioleoyllecithin bilayers has a partial molar volume of approximately zero. Biochemistry. 1985 Aug 13;24(17):4637–4645. doi: 10.1021/bi00338a024. [DOI] [PubMed] [Google Scholar]
- Kundrot C. E., Richards F. M. Crystal structure of hen egg-white lysozyme at a hydrostatic pressure of 1000 atmospheres. J Mol Biol. 1987 Jan 5;193(1):157–170. doi: 10.1016/0022-2836(87)90634-6. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Lange Y., Weinstein R. S., Steck T. L. Interaction of chlorpromazine with the human erythrocyte membrane. J Biol Chem. 1984 Jul 25;259(14):9225–9234. [PubMed] [Google Scholar]
- Nelson C. M., Schuppenhauer M. R., Clark D. S. High-pressure, high-temperature bioreactor for comparing effects of hyperbaric and hydrostatic pressure on bacterial growth. Appl Environ Microbiol. 1992 May;58(5):1789–1793. doi: 10.1128/aem.58.5.1789-1793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portlock S. H., Clague M. J., Cherry R. J. Leakage of internal markers from erythrocytes and lipid vesicles induced by melittin, gramicidin S and alamethicin: a comparative study. Biochim Biophys Acta. 1990 Nov 30;1030(1):1–10. doi: 10.1016/0005-2736(90)90231-c. [DOI] [PubMed] [Google Scholar]
- Royer C. A., Weber G., Daly T. J., Matthews K. S. Dissociation of the lactose repressor protein tetramer using high hydrostatic pressure. Biochemistry. 1986 Dec 16;25(25):8308–8315. doi: 10.1021/bi00373a027. [DOI] [PubMed] [Google Scholar]
- Trägner D., Csordas A. Biphasic interaction of Triton detergents with the erythrocyte membrane. Biochem J. 1987 Jun 15;244(3):605–609. doi: 10.1042/bj2440605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Drickamer H. G. The effect of high pressure upon proteins and other biomolecules. Q Rev Biophys. 1983 Feb;16(1):89–112. doi: 10.1017/s0033583500004935. [DOI] [PubMed] [Google Scholar]
- Zaslavsky B. Y., Ossipov N. N., Krivich V. S., Baholdina L. P., Rogozhin S. V. Action of surface-active substances on biological membranes. II. Hemolytic activity of nonionic surfactants. Biochim Biophys Acta. 1978 Feb 2;507(1):1–7. doi: 10.1016/0005-2736(78)90368-1. [DOI] [PubMed] [Google Scholar]