Abstract
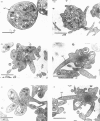
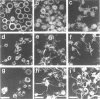
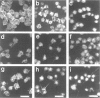
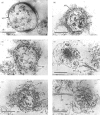
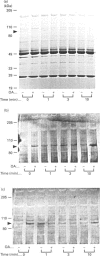
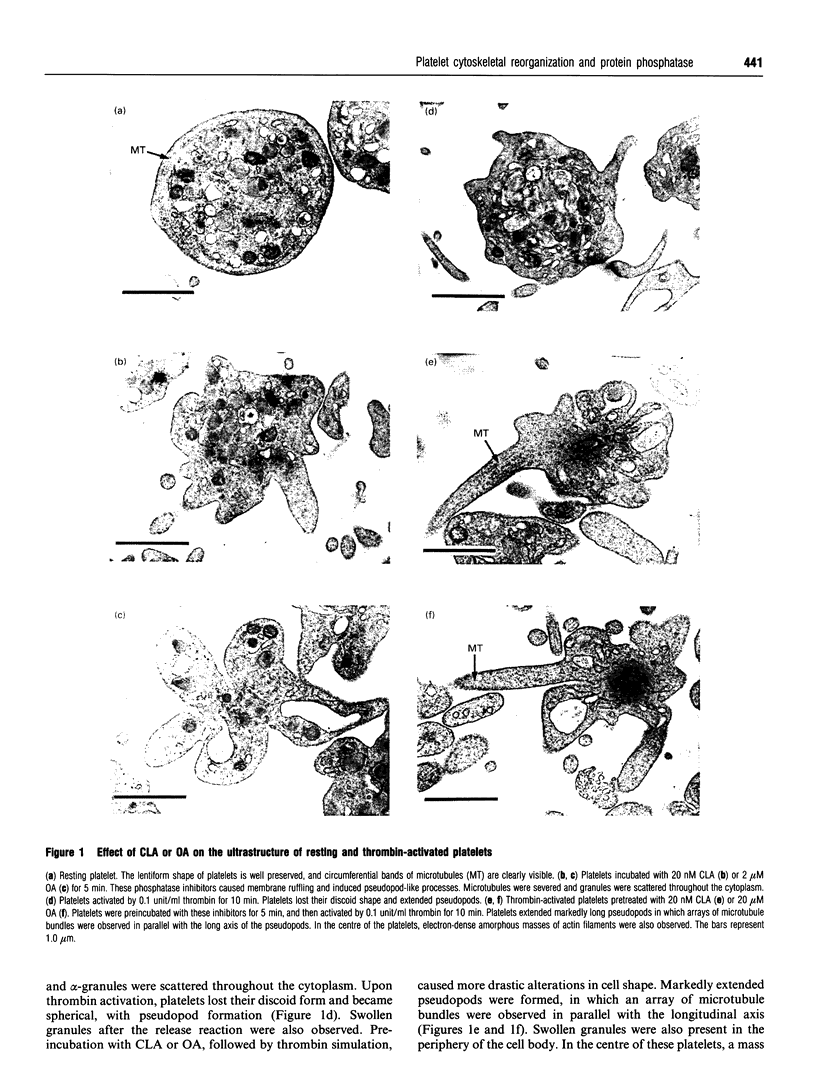
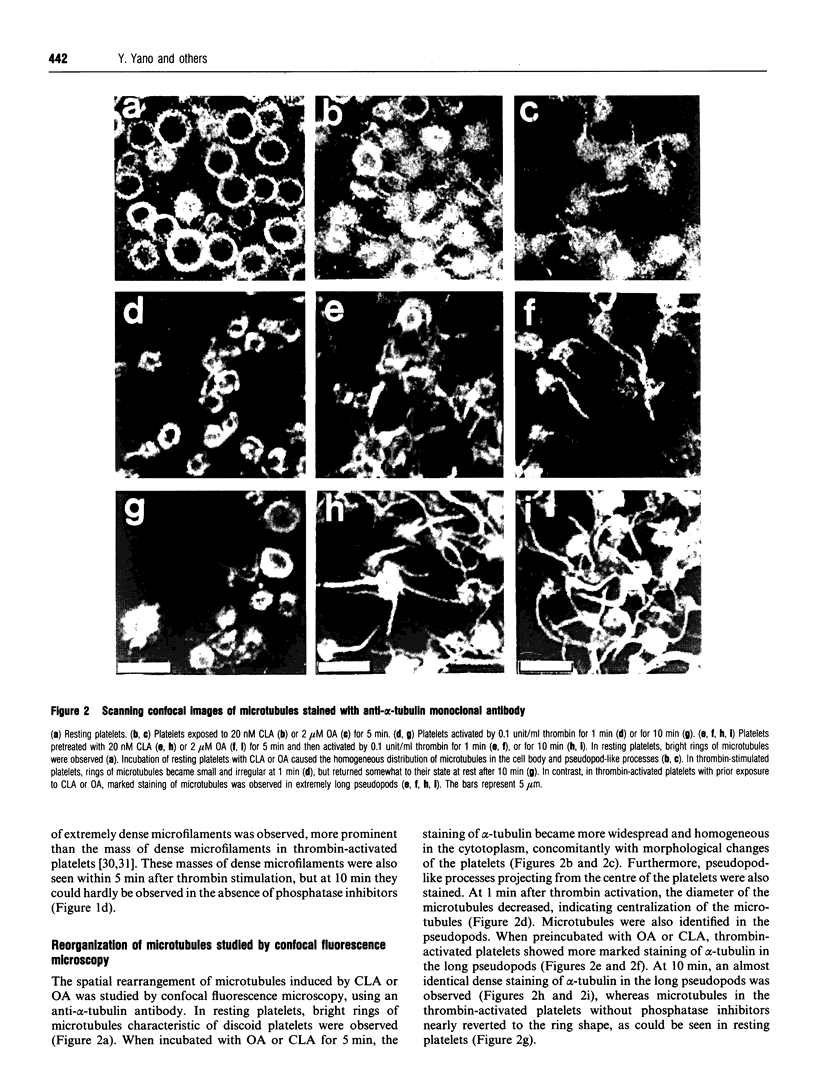
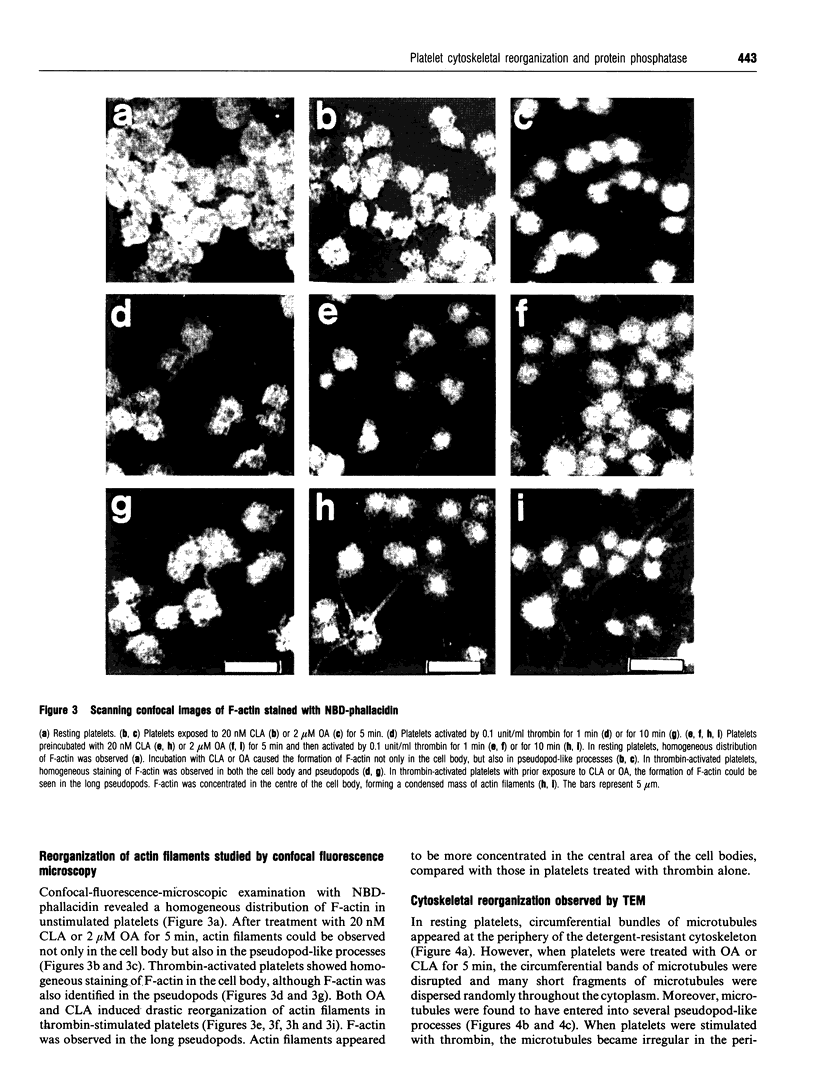
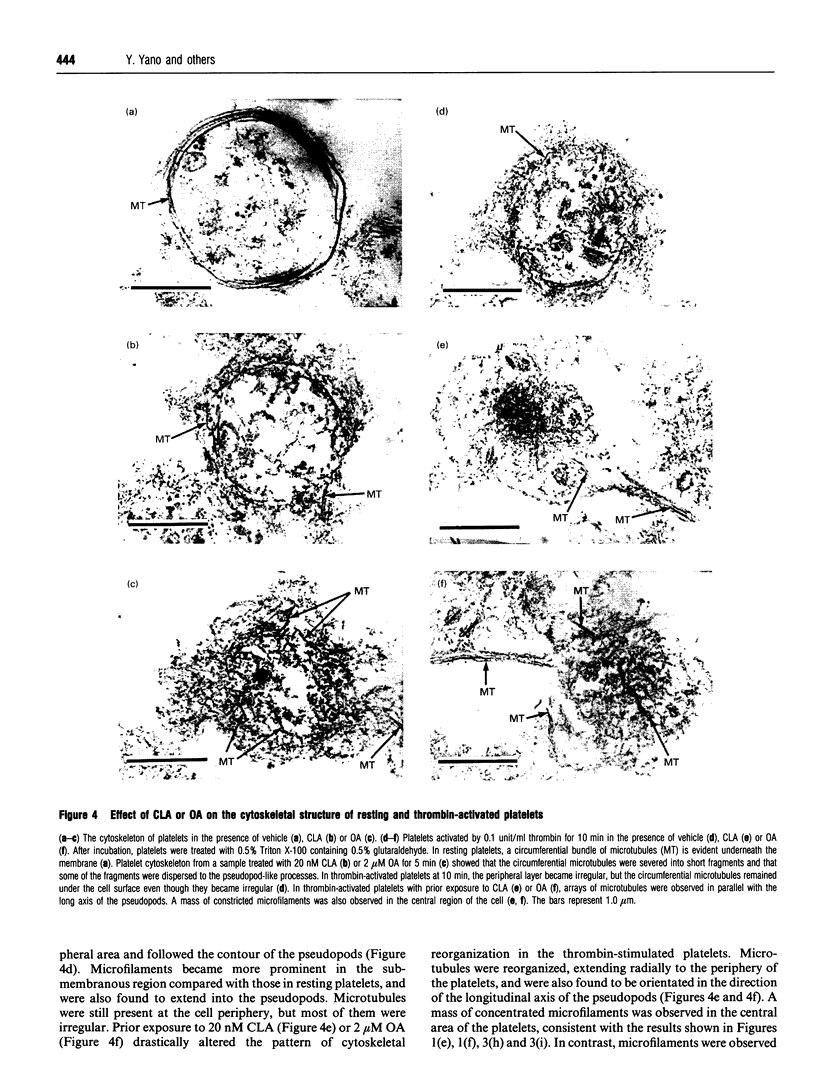
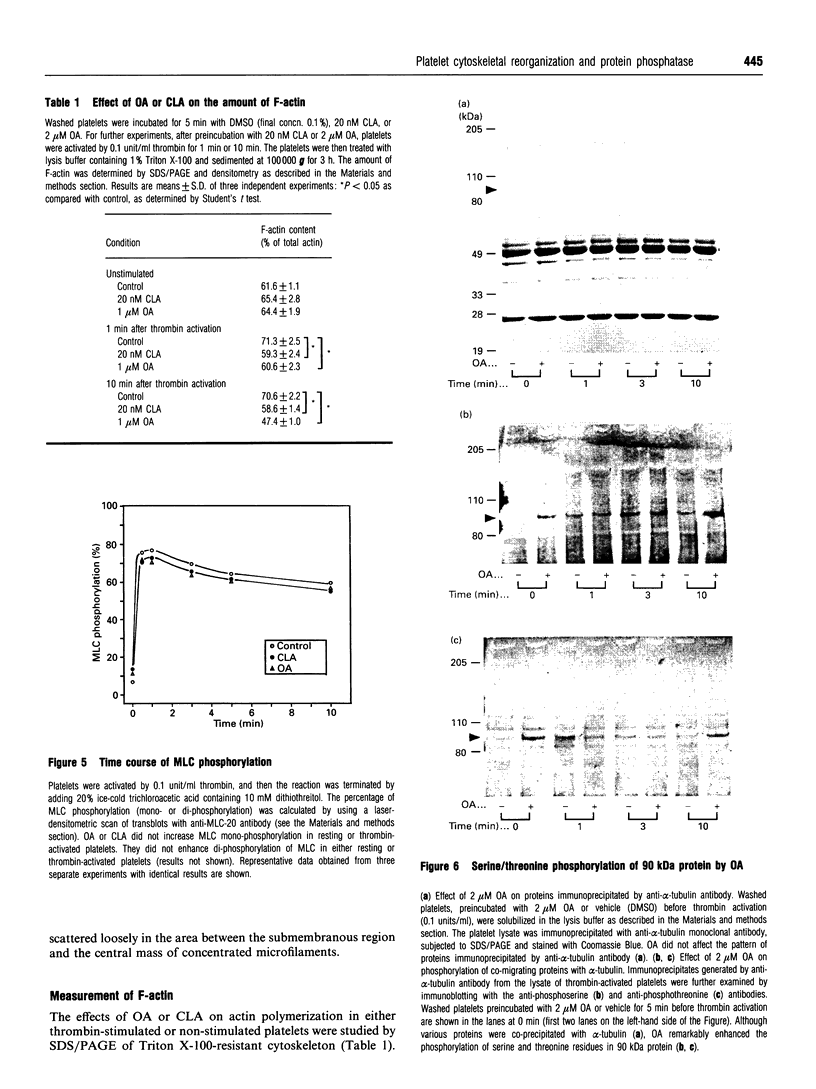
Okadaic acid (OA) and calyculin A (CLA), which are potent and specific inhibitors of serine/threonine protein phosphatases type 1 and 2A, have been shown to induce drastic changes in platelet morphology. The aim of this study was to analyse the molecular mechanisms of OA- or CLA-induced cytoskeletal reorganization, with a specific focus on microtubules and actin filaments. Confocal fluorescence microscopy revealed that OA or CLA altered the distribution of microtubules from marginal band arrangements to homogeneous patterns, consistent with the transmission-electron-microscopic finding that microtubules were fragmented and redistributed into pseudopod-like processes. In thrombin-activated platelets, OA or CLA induced extremely long pseudopods containing an array of microtubules and actin filaments, and a condensed mass of actin filaments in the centre of platelets. OA or CLA induced the constriction of actin filaments without an increase in filamentous (F)-actin, and also rather significantly inhibited actin polymerization in thrombin-activated platelets. Furthermore, neither OA or CLA enhanced phosphorylation of myosin light chain (MLC). By immunoprecipitation of platelet lysate with anti-alpha-tubulin antibody, a 90 kDa protein was co-precipitated with tubulin and was predominantly phosphorylated in the presence of OA. As the time-dependent phosphorylation of 90 kDa protein correlated well with the reorganization of microtubules, these data suggest that phosphorylation and dephosphorylation of this protein might play a role in the regulation of microtubule organization. These findings indicate that OA or CLA induces reorganization of microtubules and actin filaments via the phosphorylation of a microtubule-associated 90 kDa protein and an MLC-phosphorylation-independent mechanism. mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975 Aug 14;256(5518):597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Aksoy M. O., Mras S., Kamm K. E., Murphy R. A. Ca2+, cAMP, and changes in myosin phosphorylation during contraction of smooth muscle. Am J Physiol. 1983 Sep;245(3):C255–C270. doi: 10.1152/ajpcell.1983.245.3.C255. [DOI] [PubMed] [Google Scholar]
- Behnke O. Microtubules in disk-shaped blood cells. Int Rev Exp Pathol. 1970;9:1–92. [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. C., Butler R. G., Morris P. A., Gerrard J. M. Separable assembly of platelet pseudopodal and contractile cytoskeletons. Cell. 1982 Sep;30(2):385–393. doi: 10.1016/0092-8674(82)90236-7. [DOI] [PubMed] [Google Scholar]
- Chartier L., Rankin L. L., Allen R. E., Kato Y., Fusetani N., Karaki H., Watabe S., Hartshorne D. J. Calyculin-A increases the level of protein phosphorylation and changes the shape of 3T3 fibroblasts. Cell Motil Cytoskeleton. 1991;18(1):26–40. doi: 10.1002/cm.970180104. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Murphy R. A. Calcium-dependent stress maintenance without myosin phosphorylation in skinned smooth muscle. Science. 1983 Jul 29;221(4609):464–466. doi: 10.1126/science.6867722. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Rosevear E., Goldman R. D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. The contractile system of blood platelets and its function. Methods Achiev Exp Pathol. 1979;9:40–86. [PubMed] [Google Scholar]
- David-Ferreira J. F. The blood platelet: electron microscopic studies. Int Rev Cytol. 1964;17:99–148. doi: 10.1016/s0074-7696(08)60406-4. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. The cytoskeleton of blood platelets viewed by immunofluorescence microscopy. Eur J Cell Biol. 1981 Apr;24(1):45–52. [PubMed] [Google Scholar]
- Downey G. P., Takai A., Zamel R., Grinstein S., Chan C. K. Okadaic acid-induced actin assembly in neutrophils: role of protein phosphatases. J Cell Physiol. 1993 Jun;155(3):505–519. doi: 10.1002/jcp.1041550309. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Brautigan D. L., Vallee R., Olmsted J., Fujiki H., Goldman R. D. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11093–11097. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliksman N. R., Parsons S. F., Salmon E. D. Okadaic acid induces interphase to mitotic-like microtubule dynamic instability by inactivating rescue. J Cell Biol. 1992 Dec;119(5):1271–1276. doi: 10.1083/jcb.119.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., DeSisto M. The cytoskeleton of the resting human blood platelet: structure of the membrane skeleton and its attachment to actin filaments. J Cell Biol. 1991 Feb;112(3):407–425. doi: 10.1083/jcb.112.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon G. B., Taylor D. A. Microtubules in hamster platelets. J Cell Biol. 1965 Aug;26(2):673–676. doi: 10.1083/jcb.26.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffetz D., Fridkin M., Zick Y. Generation and use of antibodies to phosphothreonine. Methods Enzymol. 1991;201:44–53. doi: 10.1016/0076-6879(91)01007-o. [DOI] [PubMed] [Google Scholar]
- Heptinstall S., Glenn J., Spangenberg P. Changes in G-actin after platelet activation in platelet rich plasma. Thromb Haemost. 1992 Dec 7;68(6):727–730. [PubMed] [Google Scholar]
- Hirano K., Chartier L., Taylor R. G., Allen R. E., Fusetani N., Karaki H., Hartshorne D. J. Changes in the cytoskeleton of 3T3 fibroblasts induced by the phosphatase inhibitor, calyculin-A. J Muscle Res Cell Motil. 1992 Jun;13(3):341–353. doi: 10.1007/BF01766462. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Ohta K., Gotoh Y., Mori A., Murofushi H., Sakai H., Nishida E. Mitogen-activated-protein-kinase-catalyzed phosphorylation of microtubule-associated proteins, microtubule-associated protein 2 and microtubule-associated protein 4, induces an alteration in their function. Eur J Biochem. 1992 Jan 15;203(1-2):43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Itoh K., Hara T., Shibata N. Diphosphorylation of platelet myosin by myosin light chain kinase. Biochim Biophys Acta. 1992 Feb 3;1133(3):286–292. doi: 10.1016/0167-4889(92)90049-h. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., Fox J. E., Edwards H. H., Phillips D. R. Changes in the cytoskeletal structure of human platelets following thrombin activation. J Biol Chem. 1981 Jul 10;256(13):6927–6932. [PubMed] [Google Scholar]
- Kenney D. M., Linck R. W. The cystoskeleton of unstimulated blood platelets: structure and composition of the isolated marginal microtubular band. J Cell Sci. 1985 Oct;78:1–22. doi: 10.1242/jcs.78.1.1. [DOI] [PubMed] [Google Scholar]
- Kreienbühl P., Keller H., Niggli V. Protein phosphatase inhibitors okadaic acid and calyculin A alter cell shape and F-actin distribution and inhibit stimulus-dependent increases in cytoskeletal actin of human neutrophils. Blood. 1992 Dec 1;80(11):2911–2919. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerea K. M. Inhibitors of protein phosphatase type 1 and 2A attenuate phosphatidylinositol metabolism and Ca(2+)-transients in human platelets. Role of a cdc2-related protein kinase. Biochemistry. 1992 Jul 21;31(28):6553–6561. doi: 10.1021/bi00143a027. [DOI] [PubMed] [Google Scholar]
- Lerea K. M. Thrombin-induced effects are selectively inhibited following treatment of intact human platelets with okadaic acid. Biochemistry. 1991 Jul 16;30(28):6819–6824. doi: 10.1021/bi00242a003. [DOI] [PubMed] [Google Scholar]
- Levine L., Gjika H. B., Van Vunakis H. Antibodies and radioimmunoassays for phosphoserine, phosphothreonine and phosphotyrosine. Serologic specificities and levels of the phosphoamino acids in cytoplasmic fractions of rat tissues. J Immunol Methods. 1989 Nov 30;124(2):239–249. doi: 10.1016/0022-1759(89)90360-8. [DOI] [PubMed] [Google Scholar]
- Maurer D. R., Majercik M. H., Bourguignon L. Y. Effect of cyclic AMP on human blood platelets: induction of pseudopod formation and protein phosphorylation. Cell Biol Int Rep. 1988 Apr;12(4):271–288. doi: 10.1016/0309-1651(88)90072-0. [DOI] [PubMed] [Google Scholar]
- Murata K., Sakon M., Kambayashi J., Yukawa M., Yano Y., Fujitani K., Kawasaki T., Shiba E., Mori T. The possible involvement of protein phosphatase 1 in thrombin-induced Ca2+ influx of human platelets. J Cell Biochem. 1993 Apr;51(4):442–445. doi: 10.1002/jcb.2400510409. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- Nakamura F., Mino T., Yamamoto J., Naka M., Tanaka T. Identification of the regulatory site in smooth muscle calponin that is phosphorylated by protein kinase C. J Biol Chem. 1993 Mar 25;268(9):6194–6201. [PubMed] [Google Scholar]
- Oda A., Daley J. F., Cabral C., Kang J. H., Smith M., Salzman E. W. Heterogeneity in filamentous actin content among individual human blood platelets. Blood. 1992 Feb 15;79(4):920–927. [PubMed] [Google Scholar]
- Olmsted J. B. Microtubule-associated proteins. Annu Rev Cell Biol. 1986;2:421–457. doi: 10.1146/annurev.cb.02.110186.002225. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. Talin is phosphorylated on tyrosine in chicken embryo fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5507–5511. doi: 10.1073/pnas.83.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Sacher M. G., Athlan E. S., Mushynski W. E. Okadaic acid induces the rapid and reversible disruption of the neurofilament network in rat dorsal root ganglion neurons. Biochem Biophys Res Commun. 1992 Jul 15;186(1):524–530. doi: 10.1016/s0006-291x(05)80839-3. [DOI] [PubMed] [Google Scholar]
- Samiei M., Daya-Makin M., Clark-Lewis I., Pelech S. L. Platelet-activating factor- and thrombin-induced stimulation of p34cdc2-cyclin histone H1 kinase activity in platelets. J Biol Chem. 1991 Aug 15;266(23):14889–14892. [PubMed] [Google Scholar]
- Stark F., Golla R., Nachmias V. T. Formation and contraction of a microfilamentous shell in saponin-permeabilized platelets. J Cell Biol. 1991 Mar;112(5):903–913. doi: 10.1083/jcb.112.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tablin F., Reeber M. J., Nachmias V. T. Platelets contain a 210K microtubule-associated protein related to a similar protein in HeLa cells. J Cell Sci. 1988 Jun;90(Pt 2):317–324. doi: 10.1242/jcs.90.2.317. [DOI] [PubMed] [Google Scholar]
- Takai A., Bialojan C., Troschka M., Rüegg J. C. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987 Jun 8;217(1):81–84. doi: 10.1016/0014-5793(87)81247-4. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ohta H., Kanda K., Tanaka T., Hidaka H., Sobue K. Phosphorylation of high-Mr caldesmon by protein kinase C modulates the regulatory function of this protein on the interaction between actin and myosin. Eur J Biochem. 1990 Mar 30;188(3):495–500. doi: 10.1111/j.1432-1033.1990.tb15427.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991 Feb 22;64(4):827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Wills V. L. Inhibition of mitosis by okadaic acid: possible involvement of a protein phosphatase 2A in the transition from metaphase to anaphase. J Cell Sci. 1992 Jan;101(Pt 1):79–91. doi: 10.1242/jcs.101.1.79. [DOI] [PubMed] [Google Scholar]
- Verde F., Labbé J. C., Dorée M., Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990 Jan 18;343(6255):233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Vorotnikov A. V., Shirinsky V. P., Gusev N. B. Phosphorylation of smooth muscle caldesmon by three protein kinases: implication for domain mapping. FEBS Lett. 1988 Aug 29;236(2):321–324. doi: 10.1016/0014-5793(88)80047-4. [DOI] [PubMed] [Google Scholar]
- Winder S. J., Walsh M. P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990 Jun 15;265(17):10148–10155. [PubMed] [Google Scholar]
- Yano Y., Kambayashi J., Shiba E., Sakon M., Oiki E., Fukuda K., Kawasaki T., Mori T. The role of protein phosphorylation and cytoskeletal reorganization in microparticle formation from the platelet plasma membrane. Biochem J. 1994 Apr 1;299(Pt 1):303–308. doi: 10.1042/bj2990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Q. Q., Rosenberg S., Lawrence J., Stracher A. Role of actin binding protein phosphorylation in platelet cytoskeleton assembly. Biochem Biophys Res Commun. 1984 Jan 30;118(2):508–513. doi: 10.1016/0006-291x(84)91332-9. [DOI] [PubMed] [Google Scholar]