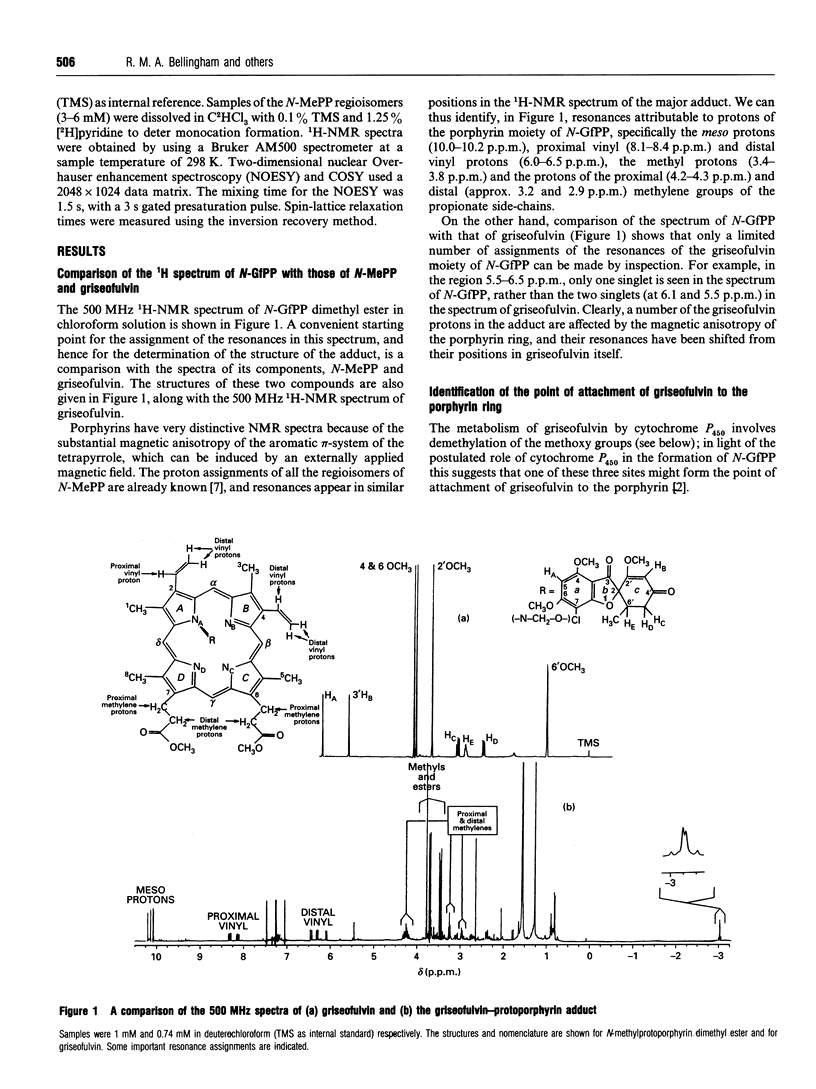
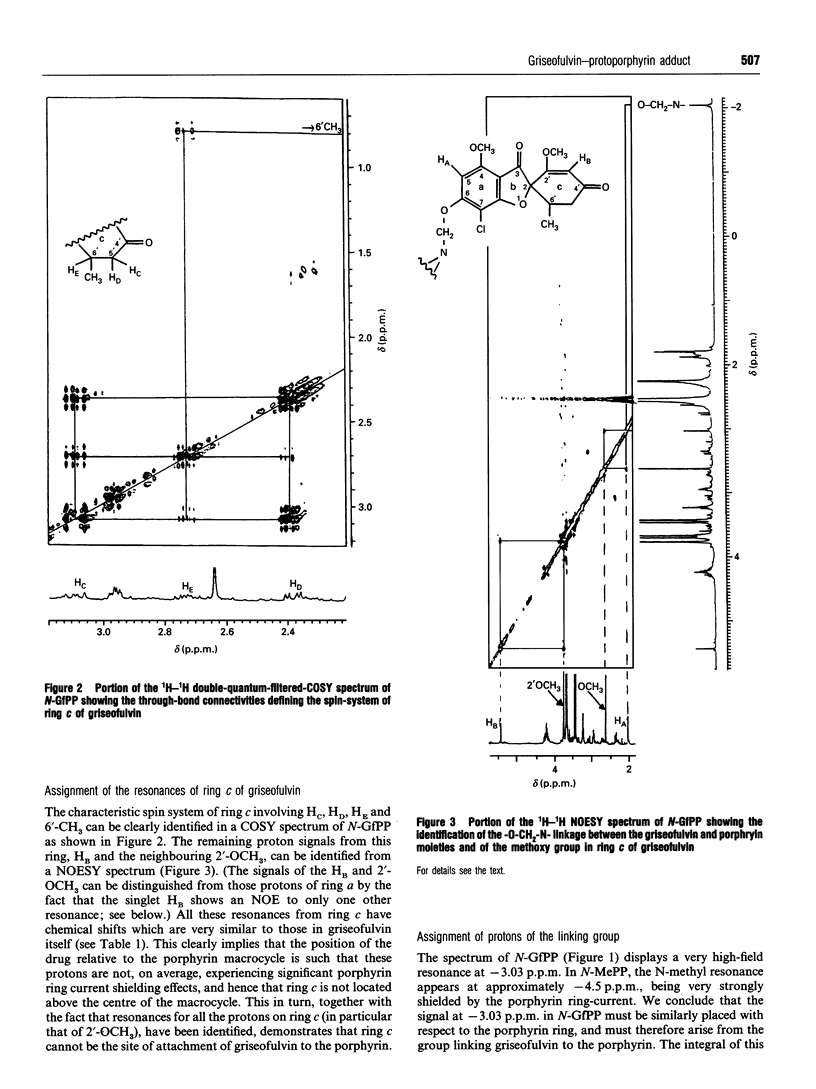
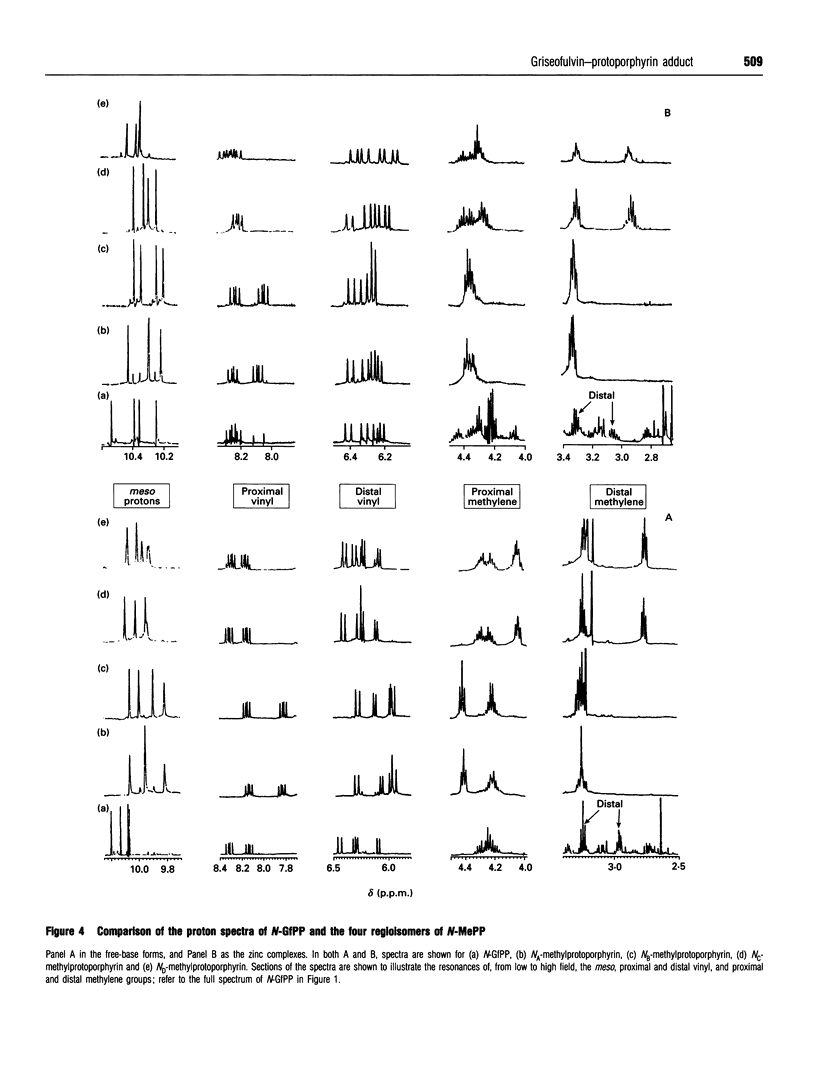
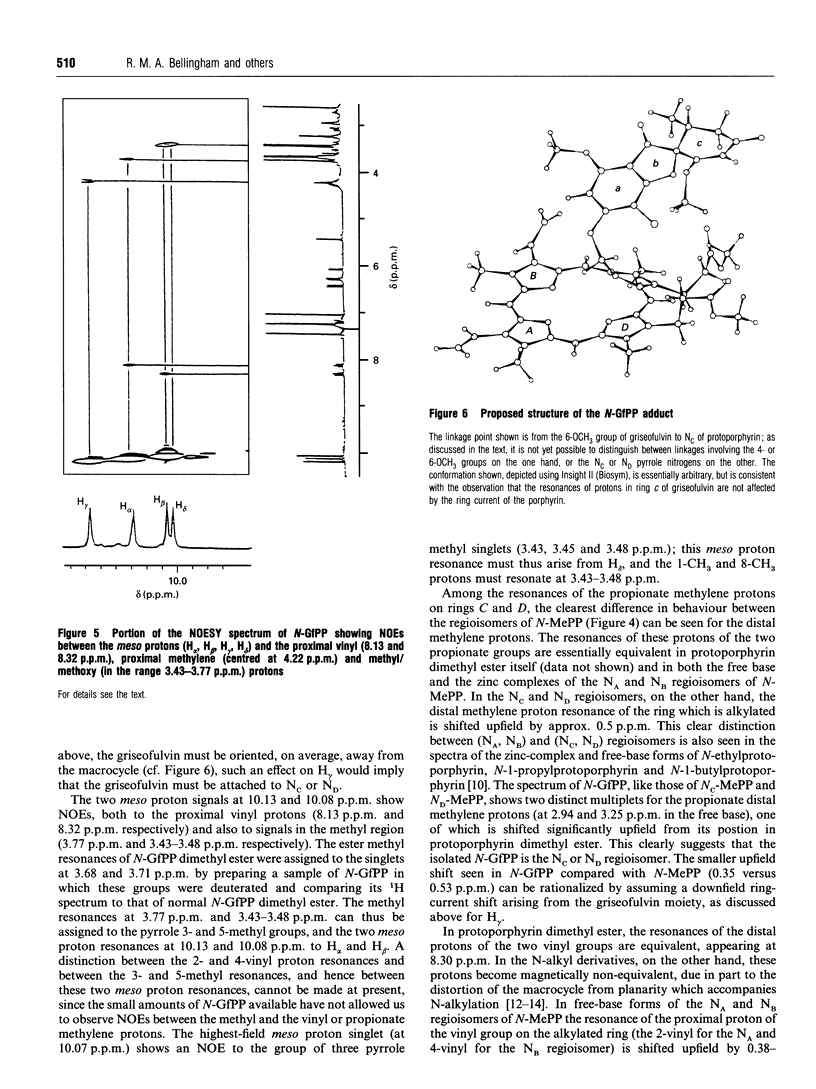
Abstract
Feeding mice with griseofulvin, a widely used anti-fungal agent which induces protoporphyria as a side-effect, leads to the formation in the liver of two green pigments which have been shown to be porphyrin adducts. In this work, the major porphyrin adduct isolated from the livers of griseofulvin-fed mice has been characterized structurally using one- and two-dimensional NMR spectroscopy. The adduct was shown to be an N-alkylated protoporphyrin IX in which the whole of griseofulvin (less a hydrogen atom) is attached to a pyrrole ring nitrogen of the porphyrin. It was shown that the drug-to-porphyrin linkage is an an -O-CH2-Npyrrole = linkage, to either the 4- or 6-position of ring a of griseofulvin. In an attempt to identify which pyrrole nitrogen is involved in this linkage, the 1H spectra of the free base and zinc complex of the adduct were compared with the corresponding spectra of the four regioisomers of N-methylprotoporphyrin. These comparisons indicated that the adduct isolated from the livers of griseofulvin-fed mice is either the NC or the ND regioisomer, although a clear distinction between these two could not be made on the available evidence. The mechanism of formation of the adduct and its relation to griseofulvin-induced protoporphyria are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusto O., Beilan H. S., Ortiz de Montellano P. R. The catalytic mechanism of cytochrome P-450. Spin-trapping evidence for one-electron substrate oxidation. J Biol Chem. 1982 Oct 10;257(19):11288–11295. [PubMed] [Google Scholar]
- DE MATTEIS F., RIMINGTON C. Disturbance of porphyrin metabolism caused by griseofulvin in mice. Br J Dermatol. 1963 Mar;75:91–104. doi: 10.1111/j.1365-2133.1963.tb13945.x. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Farmer P. B., Lamb J. H. Liver production of N-alkylated porphyrins caused in mice by treatment with substituted dihydropyridines. Evidence that the alkyl group on the pyrrole nitrogen atom originates from the drug. FEBS Lett. 1981 Jul 6;129(2):328–331. doi: 10.1016/0014-5793(81)80194-9. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Martin S. R., Milek R. L. Labelling in vivo and chirality of griseofulvin-derived N-alkylated protoporphyrins. Biochem J. 1991 Dec 15;280(Pt 3):813–816. [PMC free article] [PubMed] [Google Scholar]
- Holley A. E., Frater Y., Gibbs A. H., De Matteis F., Lamb J. H., Farmer P. B., Naylor S. Isolation of two N-monosubstituted protoporphyrins, bearing either the whole drug or a methyl group on the pyrrole nitrogen atom, from liver of mice given griseofulvin. Biochem J. 1991 Mar 15;274(Pt 3):843–848. doi: 10.1042/bj2740843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley A., King L. J., Gibbs A. H., De Matteis F. Strain and sex differences in the response of mice to drugs that induce protoporphyria: role of porphyrin biosynthesis and removal. J Biochem Toxicol. 1990 Fall;5(3):175–182. doi: 10.1002/jbt.2570050307. [DOI] [PubMed] [Google Scholar]
- Lin C. C., Magat J., Chang R., McGlotten J., Symchowicz S. Absorption, metabolism and excretion of 14C-griseofulvin in man. J Pharmacol Exp Ther. 1973 Nov;187(2):415–422. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Beilan H. S., Kunze K. L. N-Methylprotoporphyrin IX: chemical synthesis and identification as the green pigment produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine treatment. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1490–1494. doi: 10.1073/pnas.78.3.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Gibbs A. H., De Matteis F. Studies on the mechanism of experimental porphyria produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Role of a porphyrin-like inhibitor of protohaem ferro-lyase. Biochem J. 1979 Apr 15;180(1):241–244. doi: 10.1042/bj1800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Matteis F., Gibbs A. H., Jackson A. H., Weerasinghe S. Conversion of liver haem into N-substituted porphyrins or green pigments. Nature of the substituent at the pyrrole nitrogen atom. FEBS Lett. 1980 Sep 22;119(1):109–112. doi: 10.1016/0014-5793(80)81009-x. [DOI] [PubMed] [Google Scholar]
- de Matteis F., Hollands C., Gibbs A. H., de Sa N., Rizzardini M. Inactivation of cytochrome P-450 and production of N-alkylated porphyrins caused in isolated hepatocytes by substituted dihydropyridines. Structural requirements for loss of haem and alkylation of the pyrrole nitrogen atom. FEBS Lett. 1982 Aug 16;145(1):87–92. doi: 10.1016/0014-5793(82)81212-x. [DOI] [PubMed] [Google Scholar]


