Abstract
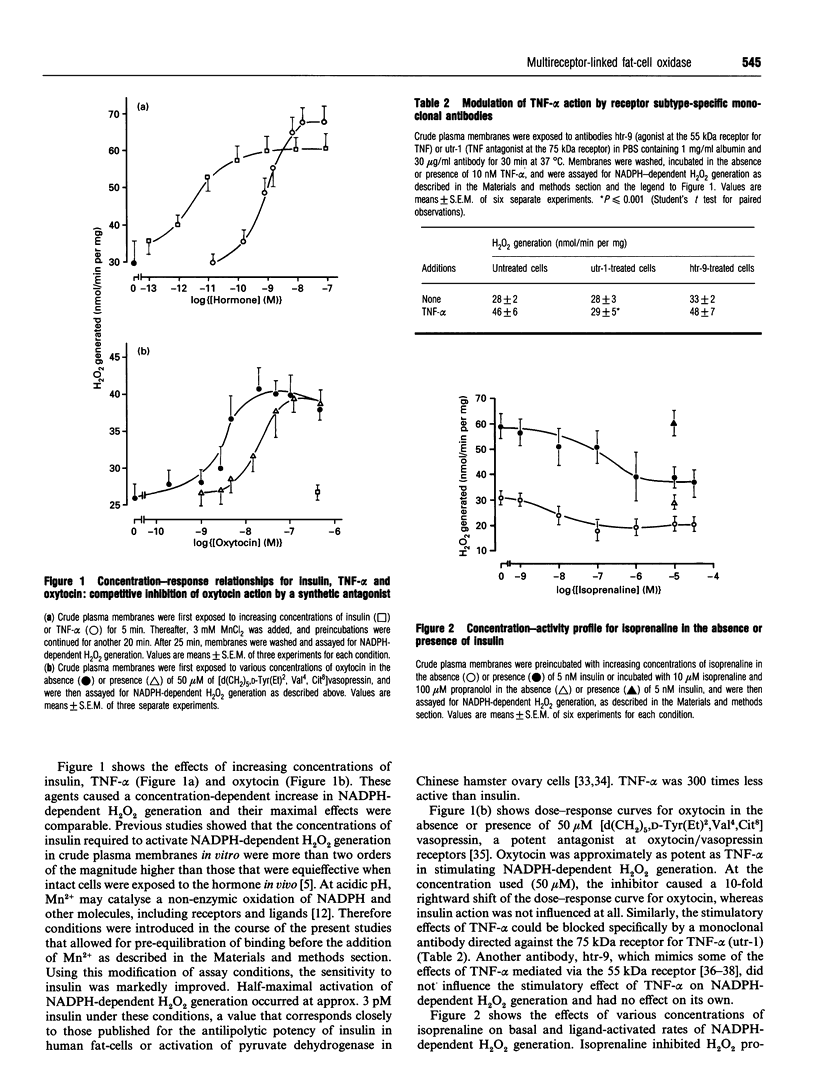
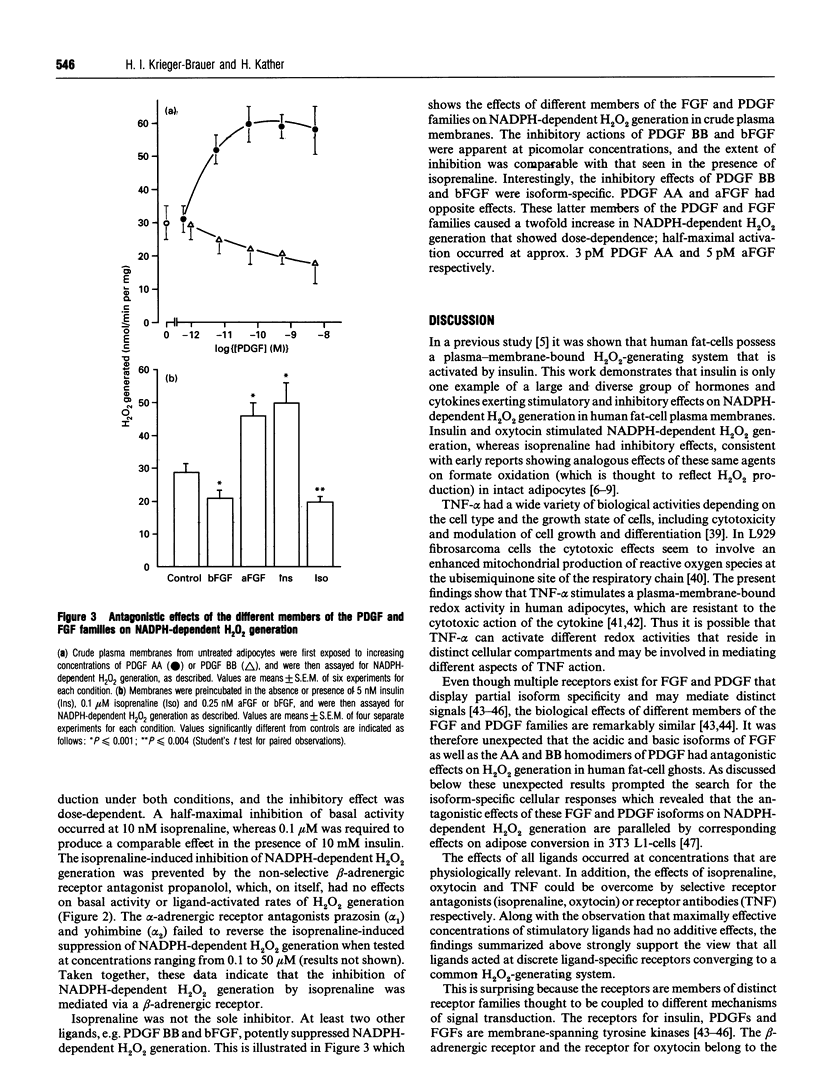
Previous work demonstrated that human fat-cells possess a plasma-membrane-bound H2O2-generating system that is activated by insulin. Here we show that this system is under antagonistic control by various hormones and cytokines that typically act through several distinct receptor families. Similarly to insulin, oxytocin and tumour necrosis factor alpha acted as stimulators of NADPH-dependent H2O2 generation, whereas isoprenaline, a beta-adrenergic agonist, had inhibitory effects. Surprisingly, the acidic and basic isoforms of fibroblast growth factor as well as homodimeric platelet-derived growth factor AA and BB had antagonistic stimulatory and inhibitory effects on NADPH-dependent H2O2 generation. The agents tested acted at discrete ligand-specific receptors and their mechanisms of action were membrane-delimited and occurred in the absence of ATP. These findings implied that established pathways of signal transduction, including receptor kinases or second-messenger-dependent protein kinases A and C, were not involved and placed the stimulus-sensitive H2O2-generating system in a position comparable with adenylate cyclase. It was concluded that the stimulus-sensitive H2O2-generating system of human fat-cells meets all criteria of a universal signal-transducing system for hormones and cytokines that may link ligand binding to cell-surface receptors to changes in the intracellular redox equilibrium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Bauskin A. R., Alkalay I., Ben-Neriah Y. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell. 1991 Aug 23;66(4):685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Boege F., Neumann E., Helmreich E. J. Structural heterogeneity of membrane receptors and GTP-binding proteins and its functional consequences for signal transduction. Eur J Biochem. 1991 Jul 1;199(1):1–15. doi: 10.1111/j.1432-1033.1991.tb16085.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus M., Schoenfeld H. J., Schlaeger E. J., Hunziker W., Lesslauer W., Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M., Brightman A. O., Lawrence J., Werderitsh D., Morré D. M., Morre D. J. Stimulation of NADH oxidase activity from rat liver plasma membranes by growth factors and hormones is decreased or absent with hepatoma plasma membranes. Biochem J. 1992 Jun 15;284(Pt 3):625–628. doi: 10.1042/bj2840625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Albano E., Tetta C., Bussolino F. The molecular action of tumor necrosis factor-alpha. Eur J Biochem. 1991 Nov 15;202(1):3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- Deme D., Virion A., Hammou N. A., Pommier J. NADPH-dependent generation of H2O2 in a thyroid particulate fraction requires Ca2+. FEBS Lett. 1985 Jul 1;186(1):107–110. doi: 10.1016/0014-5793(85)81349-1. [DOI] [PubMed] [Google Scholar]
- Desrochers P. E., Mookhtiar K., Van Wart H. E., Hasty K. A., Weiss S. J. Proteolytic inactivation of alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem. 1992 Mar 5;267(7):5005–5012. [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Garrett I. R., Boyce B. F., Oreffo R. O., Bonewald L., Poser J., Mundy G. R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990 Mar;85(3):632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk W. K. The pathway mediating insulin's effects on pyruvate dehydrogenase bypasses the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 15;266(14):8814–8819. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H. Structural and functional studies on platelet-derived growth factor. EMBO J. 1992 Dec;11(12):4251–4259. doi: 10.1002/j.1460-2075.1992.tb05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993 Jan 1;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Heyworth C. M., Whetton A. D. Mechanism of glucagon activation of adenylate cyclase in the presence of Mn2+. FEBS Lett. 1983 May 8;155(2):311–316. doi: 10.1016/0014-5793(82)80627-3. [DOI] [PubMed] [Google Scholar]
- Hurst J. K., Barrette W. C., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24(4):271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- Ikebuchi Y., Masumoto N., Tasaka K., Koike K., Kasahara K., Miyake A., Tanizawa O. Superoxide anion increases intracellular pH, intracellular free calcium, and arachidonate release in human amnion cells. J Biol Chem. 1991 Jul 15;266(20):13233–13237. [PubMed] [Google Scholar]
- Kadota S., Fantus I. G., Deragon G., Guyda H. J., Posner B. I. Stimulation of insulin-like growth factor II receptor binding and insulin receptor kinase activity in rat adipocytes. Effects of vanadate and H2O2. J Biol Chem. 1987 Jun 15;262(17):8252–8256. [PubMed] [Google Scholar]
- Kather H., Wieland E., Scheurer A., Vogel G., Wildenberg U., Joost C. Influences of variation in total energy intake and dietary composition on regulation of fat cell lipolysis in ideal-weight subjects. J Clin Invest. 1987 Aug;80(2):566–572. doi: 10.1172/JCI113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H., Wieland E., Waas W. Chemiluminescent determination of adenosine, inosine, and hypoxanthine/xanthine. Anal Biochem. 1987 May 15;163(1):45–51. doi: 10.1016/0003-2697(87)90091-1. [DOI] [PubMed] [Google Scholar]
- Kern P. A. Recombinant human tumor necrosis factor does not inhibit lipoprotein lipase in primary cultures of isolated human adipocytes. J Lipid Res. 1988 Jul;29(7):909–914. [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M. J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992 Apr 9;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kondo T., Konishi F., Inui H., Inagami T. Differing signal transductions elicited by three isoforms of platelet-derived growth factor in vascular smooth muscle cells. J Biol Chem. 1993 Feb 25;268(6):4458–4464. [PubMed] [Google Scholar]
- Koyama N., Morisaki N., Saito Y., Yoshida S. Regulatory effects of platelet-derived growth factor-AA homodimer on migration of vascular smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22806–22812. [PubMed] [Google Scholar]
- Krieger-Brauer H. I., Kather H. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem J. 1995 Apr 15;307(Pt 2):549–556. doi: 10.1042/bj3070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Brauer H. I., Kather H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J Clin Invest. 1992 Mar;89(3):1006–1013. doi: 10.1172/JCI115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledoux D., Gannoun-Zaki L., Barritault D. Interactions of FGFs with target cells. Prog Growth Factor Res. 1992;4(2):107–120. doi: 10.1016/0955-2235(92)90026-e. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Schlaeger E. J., Lahm H. W., Pan Y. C., Lesslauer W., Brockhaus M. Purification and partial amino acid sequence analysis of two distinct tumor necrosis factor receptors from HL60 cells. J Biol Chem. 1990 Nov 25;265(33):20131–20138. [PubMed] [Google Scholar]
- Malter J. S., Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991 Feb 15;266(5):3167–3171. [PubMed] [Google Scholar]
- Maly F. E., Cross A. R., Jones O. T., Wolf-Vorbeck G., Walker C., Dahinden C. A., De Weck A. L. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988 Apr 1;140(7):2334–2339. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- Mattera R., Codina J., Sekura R. D., Birnbaumer L. The interaction of nucleotides with pertussis toxin. Direct evidence for a nucleotide binding site on the toxin regulating the rate of ADP-ribosylation of Ni, the inhibitory regulatory component of adenylyl cyclase. J Biol Chem. 1986 Aug 25;261(24):11173–11179. [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore D. B., Little S. A., de Haën C. Counterregulatory control of intracellular hydrogen peroxide production by insulin and lipolytic hormones in isolated rat epididymal fat cells: a role of free fatty acids. Biochemistry. 1982 Aug 3;21(16):3886–3892. doi: 10.1021/bi00259a025. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P., Lynn W. S. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone's effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys. 1977 Nov;184(1):69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P. Mediation of the antilipolytic and lipogenic effects of insulin in adipocytes by intracellular accumulation of hydrogen peroxide. Biochem Pharmacol. 1980 May 1;29(9):1239–1246. doi: 10.1016/0006-2952(80)90280-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P., Mukherjee C. Stimulation of pyruvate dehydrogenase activity in adipocytes by oxytocin: evidence for mediation of the insulin-like effect by endogenous hydrogen peroxide independent of glucose transport. Arch Biochem Biophys. 1982 Mar;214(1):211–222. doi: 10.1016/0003-9861(82)90024-8. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O in biomembranes. Biochim Biophys Acta. 1982 Aug 11;694(1):69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente S. R., Villalba M., Schramm V. L., Rosen O. M. Mn2(+)-binding properties of a recombinant protein-tyrosine kinase derived from the human insulin receptor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2805–2809. doi: 10.1073/pnas.87.7.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccarato F., Deana R., Cavallini L., Alexandre A. Generation of hydrogen peroxide by cerebral-cortex synaptosomes. Stimulation by ionomycin and plasma-membrane depolarization. Eur J Biochem. 1989 Mar 15;180(2):473–478. doi: 10.1111/j.1432-1033.1989.tb14670.x. [DOI] [PubMed] [Google Scholar]


