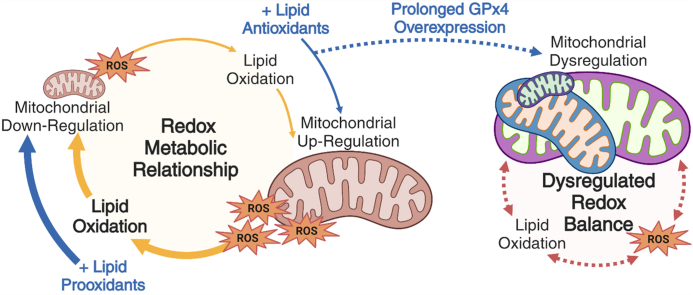
Abstract
In orthopedic research, many studies have applied vitamin E as a protective antioxidant or used tert-butyl hydroperoxide to induce oxidative injury to chondrocytes. These studies often support the hypothesis that joint pathology causes oxidative stress and increased lipid peroxidation that might be prevented with lipid antioxidants to improve cell survival or function and joint health; however, lipid antioxidant supplementation was ineffective against osteoarthritis in clinical trials and animal data have been equivocal. Moreover, increased circulating vitamin E is associated with increased rates of osteoarthritis. This disconnect between benchtop and clinical results led us to hypothesize that oxidative stress-driven paradigms of chondrocyte redox function do not capture the metabolic and physiologic effects of lipid antioxidants and prooxidants on articular chondrocytes. We used ex vivo and in vivo cartilage models to investigate the effect of lipid antioxidants on healthy, primary, articular chondrocytes and applied immuno-spin trapping techniques to provide a broad indicator of high levels of oxidative stress independent of specific reactive oxygen species. Key findings demonstrate lipid antioxidants were pro-mitochondrial while lipid prooxidants decreased mitochondrial measures. In the absence of injury, radical formation was increased by lipid antioxidants; however, in the presence of injury, radical formation was decreased. In unstressed conditions, this relationship between chondrocyte mitochondria and redox regulation was reproduced in vivo with overexpression of glutathione peroxidase 4. In mice aged 18 months or more, overexpression of glutathione peroxidase 4 significantly decreased the presence of pro-mitochondrial peroxisome proliferation activated receptor gamma and deranged the relationship between mitochondria and the redox environment. This complex interaction suggests strategies targeting articular cartilage may benefit from adopting more nuanced paradigms of articular chondrocyte redox metabolism.
Keywords: Lipid peroxidation, Glutathione peroxidase 4, Mitochondria, Cartilage, Chondrocyte, Immuno-spin trapping
Graphical abstract
Highlights
-
•
Lipid antioxidants are pro-mitochondrial and increase carbon centered radicals.
-
•
Promoting lipid peroxidation depressed mitochondrial markers and lipid peroxyl radicals.
-
•
Mitochondrial markers were increased in young glutathione peroxidase 4 overexpressing mice.
-
•
Aged mice overexpressing glutathione peroxidase 4 show dysregulation of mitochondria, the redox environment, and depressed PPAR-γ.
1. Introduction
Healthy articular cartilage is an avascular, specialized tissue that thrives under repetitive mechanical loading. After mechanical overload or injury to cartilage, resident articular chondrocytes increase oxidant production [[1], [2], [3]]. Excess oxidant production can cause mitochondrial dysfunction, diminished mitochondrial content, and, in severe cases, joint degeneration [2,[4], [5], [6], [7], [8]]. This acute mitochondrial pathology appears to involve lipid peroxidation [6] but thorough descriptions of normal intraarticular lipid peroxidation and its role in regulating chondrocyte mitochondria are lacking. Given the tremendous complexities of redox biology, and in particular lipid peroxidation, we wanted to explore the effects of lipid antioxidants and prooxidants on the mitochondria of healthy chondrocytes and examine how these manipulations altered the broader intracellular redox environment in the absence of pathology.
Paradigms in redox biology have settled on oxidative stress as a descriptor of a pathological intracellular redox balance; however, it can be unhelpful for describing changes in redox balance in the absence of pathology or stress. Conceptions of “oxidative eustress” as participating in normal cell function [[9], [10], [11]] or “oxidative distress” as an excess leading to damage and injury [12,13] have been proposed as alternative terminology to better capture normal function. For this study, we will use “intracellular redox environment” as a general descriptor of the balance of cellular oxidant production and corresponding reductive or antioxidant capacity across the many redox active species in cells. Thus, a profound oxidative shift in the normal intracellular redox environment that results in cellular stress would equate to oxidative stress. Meaningfully working within the limitations of any of these terms to rigorously relate changes in oxidation to the appearance of cellular stress (i.e. to demonstrate oxidative stress/distress) requires both specific redox measures of mechanistic species and broader redox measures that reflect deviation from the normal intracellular redox environment.
Direct assessments of the chondrocyte intracellular redox environment alongside rigorous physiological modeling of lipid peroxidation are challenging in damaged or disrupted articular joints. The example that inspired this analysis was glutathione (GSH), the most abundant intracellular antioxidant [14]. Given its abundance, GSH makes important contributions to- and is often a key indicator of the redox environment [14,15]. We note that in healthy articular cartilage, 12 % of total GSH is present as oxidized glutathione disulfide (GSSG), compared to young healthy pancreas, which contains only 1.5 % GSSG [6,16]. This level of oxidation observed in normal chondrocyte preparations under ideal conditions is more similar to damaged livers prepared and analyzed with the same techniques [17]. Rather than suggest stress, the increased GSSG in healthy chondrocytes suggests to us that chondrocytes may operate with a uniquely oxidized intracellular redox environment under healthy conditions.
This relatively high level of thiol oxidation, thus low thiol reductive capacity, implies that the oxidation of other electron rich moieties may become more favorable [18]. When considering the constituents of the cell, these oxidation targets are likely to include lipids damaged by non-enzymatic lipid oxidation via lipid peroxidation chain reactions. Many studies have shown lipid antioxidants are protective of cartilage in vitro [[19], [20], [21]], including studies from our group [6]. Studies using synovial fluid, cell lines, and OA cartilage models [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]] broadly led to the conclusion that lipid peroxidation is increased in OA or a potential contributor to OA [32,[39], [40], [41]]. Other cartilage studies related to lipid peroxidation evaluated the role of peroxisome proliferation activated receptor gamma (PPAR-γ) which is activated by several lipid peroxidation products [42] and is pro-mitochondrial [43]. These studies showed decreased PPAR-γ with increasing disease severity and that decreasing PPAR-γ expression resulted in spontaneous cartilage degeneration which was accelerated with injury [[44], [45], [46]]. These findings suggest a complex relationship between injury and cartilage health dependent upon lipid peroxidation and intracellular signaling responses. Unfortunately, the lipid antioxidant vitamin E was ineffective in clinical trials and high levels of vitamin E have been associated with increased radiographic OA [[47], [48], [49]]. This contrasts with plentiful basic research suggesting this approach might be beneficial [19,28,[50], [51], [52], [53]]. We hypothesized that analyses of injury responses and redox pathology in benchtop settings were not faithfully replicating an overarching relationship between chondrocytes’ intracellular lipid antioxidants, prooxidants, and their intracellular redox environment.
Therefore, this study sought to determine whether links between mitochondria and the chondrocyte redox environment identified in pathological settings were operative in otherwise healthy tissue. To investigate these relationships, we utilized maximally physiologically relevant in vitro models including short term cultures of large animal primary articular osteochondral tissue at physiological oxygen tension. Findings from this model were then explored in vivo in young and aged murine models with overexpression of glutathione peroxidase 4 (GPx4), a critical enzyme that removes lipid hydroperoxides, resulting in decreased lipid peroxidation [54]. We propose that chondrocytes are highly responsive to minute changes in lipid peroxidation, which functions as a critical feedback signal linking fluctuations in oxidation of the redox environment to mitochondrial regulation.
2. Materials and methods
2.1. Western blotting
To evaluate the effect of prolonged hydrogen peroxide (H2O2)-induced oxidative stress on articular chondrocytes, free swelling bovine cartilage was dosed with increasing concentrations of glucose oxidase (GO). This fungal enzyme uses glucose and oxygen to generate H2O2 [55]. Cartilage samples were dosed with 0, 0.01, 0.05, and 0.1 U/mL in normal growth media for 24 h. Samples were collected and processed for Western blot. Samples from the GO experiments were collected for assessment of peroxiredoxin (Prx) monomer/dimer formation or for mitochondrial markers translocase of outer membrane (TOMM20) and optic atrophy-1 (OPA-1). Prx preparation was done using an alkylating buffer with phosphatase inhibitors. Samples were prepared without denaturing or reducing agents and were run under non-reducing conditions [35]. For peroxisome proliferator activated receptor-γ (PPAR-γ) based measures, explants were cultured in media with either 500 nM α-tocopherol acetate (VitE), Sigma Aldrich #258024, 100 μM tert-butyl hydroperoxide (tBOOH), Thermofisher #180342500, or in combination with PPAR-γ inhibitor GW9662, Millipore Sigma #M6191, 10 μM for 4 h.
For western blotting, unless otherwise noted, fresh cartilage was removed from the bone and placed in a protease/phosphatase inhibitor cocktail then frozen at −80 °C. Samples were thawed on ice and total proteins were quantified using the BCA method (Thermo, #23221). The protein samples were denatured and reduced by addition of LDS sample buffer (Invitrogen NP0007) and DTT reducing agent (Invitrogen, NP0009) and heated at 70 °C for 10 min 25 μg of protein was loaded per well and electrophoresed through a 10 % Bis-Tris NuPage acrylamide gel (Invitrogen, NP0315), at 120 V constant by 2 h with MES-SDS running buffer (Invitrogen, NP0002). After the electrophoresis, the gel proteins were transferred to a 0.45 μM PVDF membrane (Millipore, IPVH00010), at 300 mAmp constant by 2 h with 10 % methanol, 0.1 % SDS transfer buffer (Invitrogen, NP0006-1). The transfer and protein quality were determined by staining the membrane with Ponceau S. The membrane was blocked for 30 min with 5 % bovine serum albumin (BSA). The blots were assessed for mitochondrial proteins. Primary antibodies were diluted in 2.5 % BSA. Primary antibodies were incubated overnight at 4 °C. Primary antibodies used were: PRX-2 (1:1000, Protein Tech Group, #I0664-1), PRX-3 (1:1000, Protein Tech Group, #I0664-1), OPA-1 (1:1000, Cell signaling #80471), TOMM20 (1:1000, Cell signaling #42406), GAPDH (1:1000, Santa Cruz, #0411), β-actin (1:1000, Protein Tech Group, #66009–1). The secondary antibody is HRP Goat anti-rabbit (1:1000) incubated for 1h RT (Abcam). After rinsing with TBST, the protein signals were developed and visualized using Super Signal West Femto (Thermo, 34095) and Amercham Hyperfilm ECL system (GE Healthcare, 28906839) film. Densitometry was performed in imageJ using the ponceau S staining as an indication of total protein was used as the standardized control.
2.2. Tissue culture treatments
Intact bovine and porcine stifle joints (knees) were acquired from a local abattoir approximately 1–2 h post-mortem (Bud's Custom Meats, Riverside, IA). Bovine articular osteochondral explants were harvested from the meniscus uncovered region of the tibia or taken from the load bearing region of the femur. Porcine articular osteochondral explants were taken from similar load bearing regions of both the femur and tibia. All samples were cultured in media with 45 % DMEM, 45 % F12, 10 % FBS, with amphotericin B and penicillin-streptomycin at 5 % CO2, 5 % O2, and 37 °C overnight.
To determine VitE dosing, osteochondral explants were treated with increasing concentrations of VitE. Specimens were treated with 0 nM, 100 nM, 500 nM, 1 μM, or 10 μM under normal culture conditions for 24 h then evaluated for mitochondrial staining. From this dose response, 500 nM was chosen for the subsequent experiments because it was the lowest dose to cause significant increases in mitochondrial staining. To increase lipid peroxidation, osteochondral specimens received a single dose of tBOOH. This acts to increase total lipid peroxidation [56]. To evaluate the effect of lipid prooxidants on mitochondrial content, osteochondral explants received a single treatment of tBOOH, 0 nM, 100 nM, 300 nM, 10 μM, or 100 μM, and 24 h later were evaluated for mitochondrial staining. The single dose of 100 μM tBOOH was chosen for further experiments based on the observed maximal decrease of mitochondrial staining. To ensure this decrease was not the result of toxicity of the 100 μM tBOOH dose osteochondral explants were treated with 100 μM tBOOH and 24 h after imaged for cell viability with 1 μM Calcein AM (Invitrogen, #C1430), Supplemental Figs. 1A and 1B, no toxic effect was observed at this concentration.
To evaluate the contribution of mitochondrial oxidant production associated with VitE treatment, swine osteochondral tissues were incubated overnight with or without 500 nM VitE. The next day tissue samples were treated with mitochondrial electron transport chain (ETC) inhibitor rotenone (rot, Sigma Aldrich 2.5 μM). Samples were sorted into treatment groups: control, VitE, rot, VitE + rot. After 30 min of mitochondrial inhibitor treatment all samples had 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) added to the media for a concentration of 70 mM DMPO for 1 h. After this incubation these samples were then prepared for histology.
For histological preparations, bovine and swine specimens were fixed with 10% neutral buffered formalin (NBF) with 0.3% safranin-O (saf-O) under vacuum for 4 h to preserve chondrocyte morphology [57,58]. The samples were then decalcified using 5% formic acid, which was confirmed using x-ray imaging (Faxitron). Following processing, samples were paraffin infiltrated and embedded using standard techniques [59].
2.3. Live cell imaging
All live cell imaging was performed using a Fluoview FV1000 (Olympus) confocal microscope. All samples were placed in DMEM medium without phenol red and visualized using a 20× immersion objective. Active mitochondrial content was evaluated using 1 μM MitoTracker Deep Red FM (MTDR), which binds thiol reactive chloromethyl groups [60], (Thermofisher, catalog #M22426) in media. Samples were incubated for 30 min then rinsed in phenol red free DMEM. Lipid peroxidation was evaluated using 5 μM BODIPY 581/591C11 (Thermofisher, catalog #D3861). The BODIPY probe is oxidized by lipid peroxyl radical [61]. This dye produces distinct reduced and oxidized fluorescent signals. Samples were stained with 5 μM BODIPY in DMEM without phenol red. Samples were incubated for 30 min then rinsed in and returned to phenol red free DMEM. Images were captured of the tissue depth and restricted to the meniscus uncovered loaded area. Three images were captured and the mean fluorescent intensity for each channel was analyzed in ImageJ. The mean intensity was reported as a ratio of oxidized to reduced probe.
2.4. Impact injury model
Swine specimens from the tibial plateau were subjected to mechanical impacts within a well-characterized, energy-controlled impact model [3,6,59]. Briefly, samples were allowed to recover overnight after extraction then placed in either fresh medium or medium containing 500 nM VitE for 24 h. For immuno-spin trapping (IST) measures of in situ protein radicals, 1 h prior to impact 70 mM DMPO was added to the media as previously described [59]. All samples were impacted using a custom drop tower which delivers a reproducible 2 J/cm2 impact with a stainless-steel flat, impermeable platen. Samples were returned to their respective media and cultured for 24 h then processed for histological analyses.
2.5. Animal care
All animal experiments were approved by The University of Iowa Institutional Animal Care and Use Committee under protocol #3062034 and all associated regulations were followed during these experiments. Mice were housed up to five in a cage with access to water, standard chow, and a 12 h light/dark cycle. Each group had a minimum of 10 mice consisting of both male and female mice. Mice were excluded if they were found dead in pen, otherwise all mice were included in the study. The left hind leg of each mouse was processed and analyzed by histology.
To modulate lipid peroxidation directly in vivo, C57B6J mice with transgenic overexpression of GPx4 (TgGPx4) via genomic insertion of the human GPx4 gene were compared to wild type (WT) littermates on all experimentation. TgGPx4 mice have been shown to have 3-fold increases in GPx4 expression throughout the body [54] and are mildly resistant to some non-orthopedic pathologies [54,62]. Genotyping from ear punches was done using the following primers: Forward: GAA CTT CAC CAA GGT AAG GGG GCT GTG; Reverse: CCT TCT CTA TCA CCT GTC GGG GAG GAA.
For IST (below) measures in the murine model, DMPO (0.5 mg/kg) was delivered by intraperitoneal injection 3 h before euthanasia [63,64]. The stifles were harvested, degloved and muscle removed. After harvest, the stifles were tied in an anatomic position with thread then placed in 10 % NBF and put under vacuum for 4 h. The stifles were then decalcified using 5 % formic acid. Decalcification was confirmed using x-ray imaging (Faxitron). Murine samples were then processed and embedded in a sagittal orientation to expose the medial compartment. All samples were sectioned at 5 μm. Samples which were sectioned past the area of interest were excluded from the study.
2.6. Histological staining
Several different immunohistochemical (IHC) approaches were used in this study. First, we used an automated staining system (Discovery Ultra, Roche) to visualize: mitochondrial marker TOMM20, protein glutathionylation (PSSG), and PPAR-γ. These protocols were optimized for use in brightfield via 3,3′-diaminobenzidine (DAB) or for immunofluorescence (IF) using red fluorophore cyanine 5 (cy-5) as noted. No counter stain was used for brightfield images. For the IF samples, tissue autofluorescence at 488 nm was used as a counterstain.
IHC for lipid peroxidation indicator 4-hydroxynonenal-modified proteins (4HNE) was conducted by hand. Slides were deparaffinized then placed in deionized water at 55°C overnight. After the slides were cooled, they were placed in 250 mM sodium borohydride for 15 min [16]. This preprocessing helps to unmask any 4HNE which has undergone Schiff base reactions. Primary, secondary, and developing were all applied as described below.
IST was used for detection of biomolecular radicals as an unbiased indicator of a highly oxidizing intracellular redox environment causing damage, i.e. oxidative stress/distress. This technique labels carbon centered radicals upon proteins and other large molecules that might be retained during histological processing [65,66]. This reveals areas with very highly oxidizing conditions. The technique requires the administration of DMPO prior to fixation so the DMPO can covalently bond with these radicals. IST slides were deparaffinized, placed in a citric acid antigen retrieval at 55°C overnight, then rinsed with deionized water.
Both hand stained IHC and IST methods followed the remaining steps. Endogenous peroxidases were quenched using 3% hydrogen peroxide. Samples were blocked for 45 min with a normal goat blocking solution: 88.5% PBS, 10% normal goat serum, 1% cold water fish gelatin 9% in PBS, and 0.05% tween 20. IST samples were blocked normal goat blocking solution without BSA. Slides were then incubated with primary antibody: IST- (1:150) chicken anti-DMPO (a gift from Dr. Ronald Mason), IHC- (1:400) rabbit anti-4-hydroxynonenal (Abcam, ab46545) overnight at 4°C [59,[65], [66], [67], [68]]. After rinsing the primary antibody, blocking solution was reapplied for 30 min. This was followed by incubation at room temperature for 45 min with the secondary antibody (1:200) as appropriate, goat anti-chicken for IST (Abcam, ab97135), goat anti-rabbit (Vector laboratories, #BA-1000). The slides were rinsed and incubated in Vectastain Elite ABC kit (Vector laboratories, #PK-6100) and developed with DAB substrate kit (Vector laboratories, #4100). All slides were then dehydrated, and cover slipped.
IHC and IST slides were digitized using either an Olympus VS120 or VS200. Three images were selected from the meniscus uncovered loaded region. These images were analyzed in ImageJ for intensity, or the cells were counted for positivity. IF slides were digitally imaged using an Olympus FV1000 confocal microscope with a 20× objective. For the murine samples, images were restricted to the meniscus uncovered tibia. To capture the loaded area, two images were captured without overlap and averaged to represent the loaded region. Swine images were captured at three locations in the loaded area. All image analysis was restricted to the cartilage and mean intensity/area was reported.
2.7. Statistics
Image analysis of DMPO images was done by cell counting by trained blinded graders. Mitochondrial ETC inhibitors are known to increase oxidant production so normalized to their appropriate paired control. The change in DMPO positive cells was reported.
Data were graphed using Graph Pad Prism 9 with standard error of the mean represented for groups with repeated measures and standard deviation represented for groups with single measures. Outliers were determined using ROUT analysis in Graph Pad Prism 9. Significance was determined using tests as delineated for individual experiments in the results section.
3. Results
3.1. Lipid antioxidants and prooxidants have opposite effects upon chondrocyte mitochondria
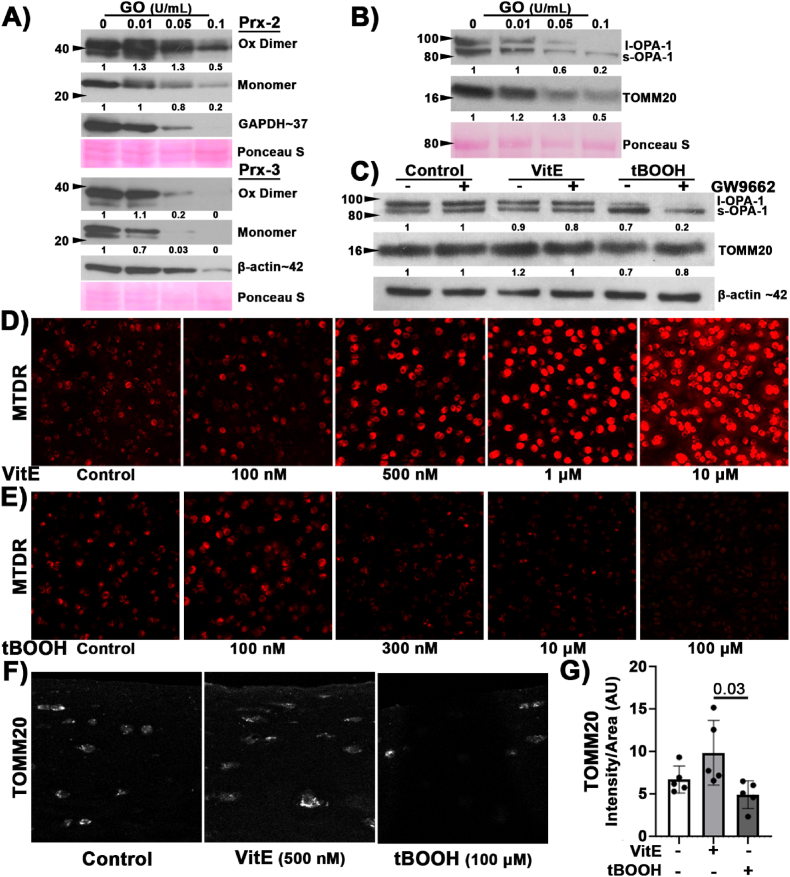
Prior characterizations of chondrocyte oxidant responses focused on menadione-driven oxidative stress within mitochondria specifically for purposes of localization [35]. We chose to use glucose oxidase (GO) supplementation in culture media to provide an extracellular but sustained and specific source of hydrogen peroxide (H2O2) in vitro. H2O2 has a relatively large diffusion distance and long half-life and thus represents a useful species for modeling sustained increases in oxidation of the intracellular redox environment over time. First, bovine articular cartilage was incubated for 24 h with increasing concentrations of GO. Then Prx2 and Prx3 were examined using non-denatured and non-reduced techniques as described [35,69]. Under these conditions, Prx2 showed a modest increase in formation of oxidized dimers at 0.01 U/mL of GO. As the GO concentration increased, we observed decreased dimer and we did not observe clear indications of increased hyper-oxidized monomer appearing at higher doses, Fig. 1A. Prx3 was also evaluated and under these concentrations we observed a decrease in both the oxidizer dimer and monomer, Fig. 1A. We did not observe a return of either Prx monomer even at toxic concentrations. This model of healthy cartilage exposed to a relatively gentle but sustained stress suggests other redox responses beyond hyper-oxidation of Prx2 and Prx3 might be responsive to prolonged increases in oxidation in the absence of injury.
Fig. 1.
Lipid antioxidants increased mitochondrial markers while lipid prooxidants decreased mitochondrial markers. (A) Bovine cartilage was treated with increasing concentrations of GO to generate H2O2. Prx2 has a modest increase in oxidized dimer followed by a decrease in both the dimer and monomer with increasing concentrations of GO; whereas Prx3 had decreased dimer and monomer with increasing concentration of GO, n = 3. (B) Representative western blots with corresponding Ponceau s stain (red, loading control) illustrated increased GO concentration coincided with a change to OPA-1 and decreased TOMM20, n = 3. (C) Representative western blot of l-OPA-1 to s-OPA-1 isoform change after 4 h VitE (500 nM) or tBOOH (100 μM) treatment, n = 3. (D) Bovine osteochondral explants treated for 24 h with increasing concentrations of VitE. Mitochondria, visualized using MTDR, increased in a dose dependent manner. 20× objective. (E) Bovine osteochondral explants were treated with increasing concentrations of tBOOH. 24 h after treatment mitochondria were visualized with MTDR. As tBOOH increased, we observed a decrease in MTDR staining intensity. 20× objective. (F) Representative images of the superficial articular cartilage stained for TOMM20 (white) after 24 h of VitE or tBOOH. 40× objective. (G) TOMM20, staining intensity trended higher after 24 h treatment with VitE and lower 24 h after tBOOH. VitE and tBOOH groups were significantly different. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To better describe the mitochondrial effects of this sustained increase in oxidation, we investigated the effects of increasing GO concentrations upon the mitochondria with outer mitochondrial translocation marker TOMM20 and the key mitochondrial regulator OPA-1. OPA-1 participates in mitochondrial quality control [70] and facilitates mitochondrial cristae organization [[70], [71], [72], [73], [74]]. The long isoform (l-OPA-1) is associated with normal or healthy cristae and is cleaved to produce the short isoform (s-OPA-1) under stressed conditions. Increased presence of s-OPA-1 is associated with oxidative stress [72] and loss of mitochondrial membrane potential [73]. With increasing GO concentration, we observed a change in the predominant isoform of OPA-1 from l-OPA-1 to s-OPA-1, coinciding with a decrease in TOMM20, Fig. 1B. To increase the specificity of our applied oxidative stress, we modulated lipid peroxidation using well characterized effectors VitE and tBOOH at an acute timepoint of 4 h after treatment. VitE showed no observable effects on OPA-1 between the control and VitE treated groups. However, 4 h of exposure to tBOOH induced s-OPA-1, Fig. 1C. There was no clear additional effect of PPAR-γ inhibitor GW9662 (10 μM) treatment after tBOOH exposure. These results support a connection between lipid peroxidation and mitochondrial downregulation distinct from the “floodgate hypothesis” of the peroxiredoxin system, wherein peroxiredoxins can become hyperoxidized triggering rapid signaling of oxidative stress responses [75]. Rather than a rapid response to stress, data suggest an ongoing reciprocal interaction between lipid peroxidation and mitochondria.
Although VitE did not appear to alter mitochondrial markers at the 4 h timepoint, we were interested if supplementing lipid antioxidants for a longer time would increase chondrocyte mitochondrial content in the absence of injury. Bovine osteochondral explants were incubated for 24 h in either 0 nM, 100 nM, 500 nM, 1 μM, or 10 μM VitE. Mitochondrial content was evaluated using live cell imaging with MTDR. We observed a dose dependent increase in MTDR staining with increased concentrations of VitE and note the low levels of VitE needed to observe an increase in MTDR, Fig. 1D. The 500 nM VitE dose was used for subsequent experiments because this dose caused an increase in MTDR without causing saturation or extracellular staining observed at higher doses. Mitochondrial content was also assessed 24 h after increasing concentrations of tBOOH. As tBOOH increased there was a decrease in MTDR staining intensity, Fig. 1E. The 100 μM dose of tBOOH was chosen for subsequent experiments because it maximally diminished MTDR staining intensity with no observed toxicity, Supplemental Figs. 1A and 1B. Although MTDR is described as minimally sensitive to changes in mitochondrial membrane potential, MitoTracker dyes generally can be affected by membrane potential [60]. Thus, we also evaluated a membrane potential independent marker of mitochondria, using IHC to identify mitochondrial marker TOMM20. TOMM20 intensity trends higher with VitE treatment and lower with tBOOH and VitE and tBOOH are significantly different in terms of TOMM20 content, Fig. 1F and G. These data show that increasing or decreasing lipid peroxidation has inverse effects on mitochondria.
3.2. Incubation with lipid prooxidants led to a more reduced intracellular redox environment
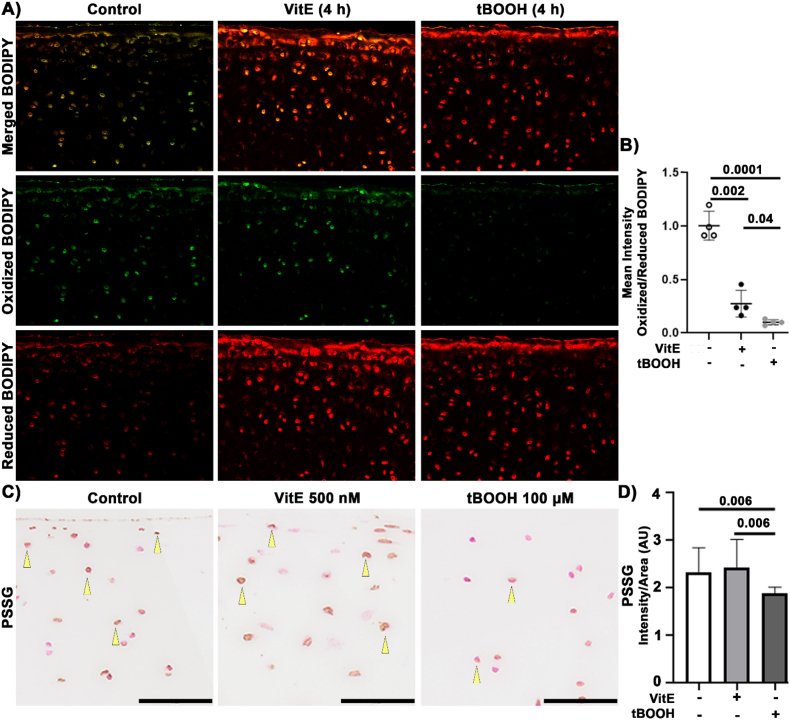
Following this observation, we wanted to test if the mitochondrial changes observed 4 h after tBOOH incubation were associated with increased lipid peroxidation. To test this, we used the lipid peroxidation-sensitive live cell dye BODIPY C11 [61]. BODIPY is a red fluorescent lipophilic stain that fluoresces green when oxidized by lipid peroxyl radicals critical to lipid peroxidation chain reactions, Fig. 2A. This technique was used on osteochondral explants 4 h after either VitE or tBOOH. We used the mean cell intensity to calculate the ratio of oxidized to reduced BODIPY, Fig. 2B. As anticipated, the VitE treatment showed less BODIPY oxidation than controls, Fig. 2A. Contrary to expectations, 4 h after tBOOH showed the least oxidized BODIPY, Fig. 2A. This suggests that acute increases in lipid oxidation led to altered redox function, specifically the loss of basal lipid peroxyl radical formation from chondrocytes despite an absence of toxicity from the tBOOH.
Fig. 2.
Lipid Prooxidants Decrease Peroxyl Radical Formation and Protein Glutathionylation. Porcine osteochondral explants were incubated for 24 h with either 500 nM VitE or 100 μM tBOOH. (A) Representative images of BODIPY in articular cartilage. 20× objective. (B) The mean intensity ratio of oxidized:reduced shows that the control specimens had the most oxidized BODIPY. VitE decreased BODIPY and tBOOH had the least oxidized BODIPY, significantly less than both other groups. (C) Representative images of PSSG staining. Bar represents 50 μm. (D) The mean intensity of PSSG was unchanged with VitE treatment. PSSG intensity decreased after tBOOH treatment. Swine osteochondral explants were incubated for 4 h with either 500 nM VitE or 100 μM tBOOH, n = 4.
Because mitochondria are the primary source of oxidants in chondrocytes [5], we wondered whether decreases in mitochondria resulting from tBOOH would further alter the intracellular redox environment. Porcine osteochondral explants were cultured with either VitE or tBOOH for 24 h and evaluated for protein-thiol oxidation using a PSSG-recognizing antibody. GSH can be adducted to protein spontaneously or in association with protective functions against protein misfolding, aggregation, and hyper-oxidation of sulfhydryl moieties. We observed a similar amount of PSSG between control and VitE samples, Fig. 2C and D. Interestingly, despite tBOOH being a prooxidant there was a decrease in PSSG, Fig. 2D. This coheres well with results from BODIPY staining and suggests that lipid prooxidants reduce the oxidation of protein thiols, decreasing protein-glutathione disulfide formation 20 h after decreases in mitochondria and lipid peroxyl radical were observed. These data suggest that even small increases in lipid peroxidation can result in rapid downregulation of mitochondria and a subsequent decrease in oxidation across the intracellular redox environment.
3.3. VitE increases IST in the absence of injury but decreases IST after injury
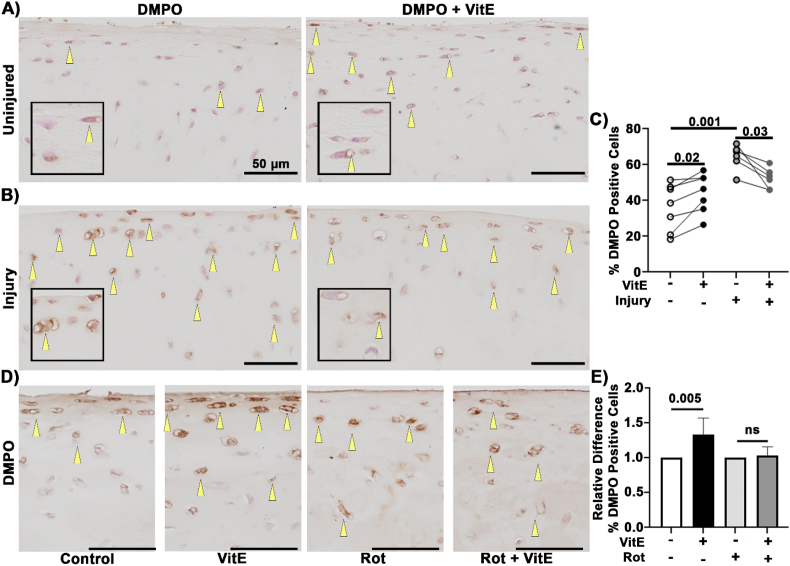
To better understand how lipid antioxidants were affecting the intracellular redox environment, we evaluated cartilage radical formation using IST [59,66]. This technique uses DMPO to reveal protein radical formation in tissue, providing a broad indicator of a highly oxidizing environment (i.e. oxidative stress/distress) that is not dependent upon specific radical species. IST reveals the total radical content on any component of the cell retained through fixation, i.e. radicals on macromolecules like DNA and protein [67,76]. For the ex vivo study, samples were incubated with DMPO or DMPO with VitE for 24 h before processing for histology. The control samples had DMPO positive cells in the transitional zones and near the articular surface, Fig. 3A. The controls had an average of 40% DMPO positive cells, Fig. 3C. Interestingly, VitE samples had greater intensity in the cells that stained positive for DMPO, Fig. 3A. The VitE samples also had higher percentage DMPO positive cells compared to paired controls, Fig. 3C. This suggests that the basal increase in mitochondria caused by VitE also included increased intracellular radical formation and contributed to a more oxidizing intracellular environment in the absence of injury.
Fig. 3.
Lipid antioxidants increased basal IST but decreased IST after injury. Yellow arrows indicate positive staining for DMPO. (A) Representative images of DMPO staining in uninjured cartilage with DMPO and DMPO with VitE. (B) Representative images of DMPO staining 24 h after injury with DMPO and DMPO with VitE. (C) VitE increased DMPO staining in the absence of injury. Paired non-parametric t-test results shown. VitE shows lower DMPO staining after injury compared to the DMPO control. Impact increased DMPO staining in untreated tissue via unpaired non-parametric t-test. (D) Representative images of DMPO staining in samples treated with VitE or in combination of mitochondrial inhibitor Rot (2.5 μM). Scale bar is 50 μm. (E) Relative difference between % positive DMPO and treatment controls. VitE significantly increased the % positive DMPO cells. Addition of mitochondrial inhibitor Rot ablated this response to VitE, n = 6, unpaired t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To assess this same endpoint in the presence of injury-associated damage, we utilized a previously characterized injury model [6,77,78] to trigger acute, damaging oxidation. Injury increased the DMPO positive cells at the articular surface while also increasing their intracellular intensity, Fig. 3B and C. This finding agrees with previous studies which demonstrate increased oxidant production after mechanical injury [1,2,6]. In the case of injury with VitE treatment the %DMPO positive cell were significantly decreased compared to impact alone, Fig. 3B and C. Taken together, these results demonstrate that intracellular radical production increased in response to a radical scavenging antioxidant in the absence of injury; however, that same antioxidant decreased radical production from mechanical injury.
To determine whether increased protein radicals in the presence of VitE are linked to mitochondrial ETC function, osteochondral explants were incubated overnight with VitE (500 nM) followed by treatment with complex I inhibitor Rot (2.5 μM) for 30 min before the administration of DMPO (70 mM). Representative images demonstrate that VitE increases the number of cells staining positive for DMPO whereas Rot treatment rendered chondrocyte mitochondria unresponsive to VitE, Fig. 3D and E. These results support the hypothesis that mitochondrial activity is an important contributor to lipid peroxidation and protein radical formation in this setting.
3.4. GPx4 overexpression increased mitochondrial TOMM20 content and altered the redox environment in vivo
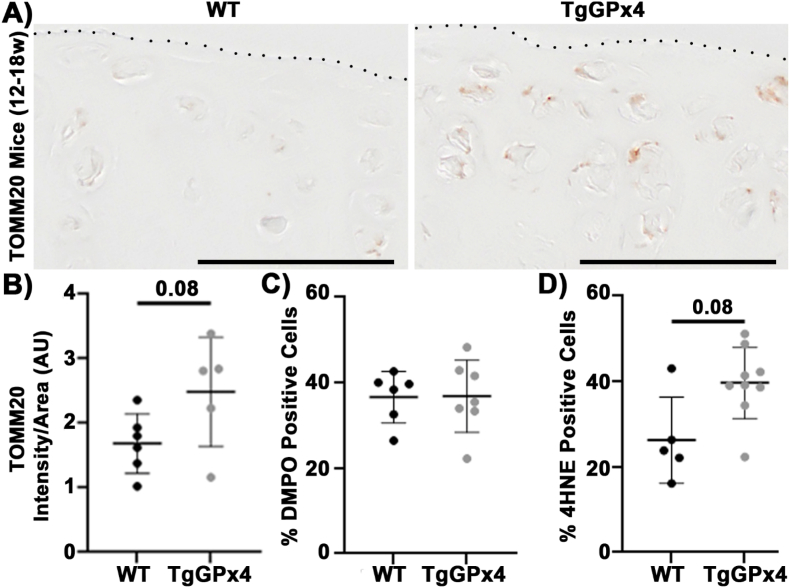
Given the observed sensitivity of primary chondrocytes to lipid peroxidation, we sought to manipulate intracellular lipid peroxidation as rigorously as possible to establish the effects of altering lipid peroxidation in normal chondrocytes. GPx4 is an important enzyme for the removal of lipid hydroperoxides [54], thus it functions similarly to VitE in opposing lipid peroxidation [18]. We first evaluated TgGPx4 mice, which possess an extra genomic copy of the enzyme, at 12–18 weeks of age. IHC staining of the meniscus uncovered, load bearing region of the tibia showed more TOMM20 staining in the TgGPx4 mice, Fig. 4A and B. Thus, augmenting lipid antioxidants in vivo had a similar effect to the ex vivo model, increasing chondrocyte mitochondria in young, healthy mice.
Fig. 4.
TgGPx4 increased TOMM20 content and minimally altered the redox environment of healthy articular chondrocytes. (A) Representative images of TOMM20 from WT and TgGPx4 mice demonstrate TOMM20 was increased in TgGPx4 mice (12–18 w). Dotted line denotes the articular surface, scale bar 50 μm. (B) TOMM20 intensity trends higher in TgGPx4 compared to WT. (C) Mice (12–18 w) had no difference in %DMPO positive cells between the WT and TgGPx4. (D) 4HNE trended higher in with TgGPx4 compared to WT.
Next, the intracellular redox environment of these TgGPx4 mice was assessed using IST and IHC of 4HNE-modified proteins. In contrast to IST which denotes protein radicals, 4HNE modification is a common by-product of aldehydes produced by lipid peroxidation of arachidonic acid. Despite increased mitochondria, TgGPx4 mice did not show a difference in %DMPO positive cells compared to WT, Fig. 4C. This suggests that there was not an increase in protein radicals or oxidative stress/distress in the chondrocytes of young healthy mice with augmented GPx4 activity. However, 4HNE increased in the TgGPx4 mice to a similar extent as TOMM20, Fig. 4D. Thus, increased GPx4 led to increased mitochondrial content and increased indications of lipid peroxidation by-products but not oxidative stress.
3.5. Aged TgGPx4 chondrocytes demonstrate low expression of PPAR-γ, mitochondrial dysregulation, and high variance in intracellular redox environment
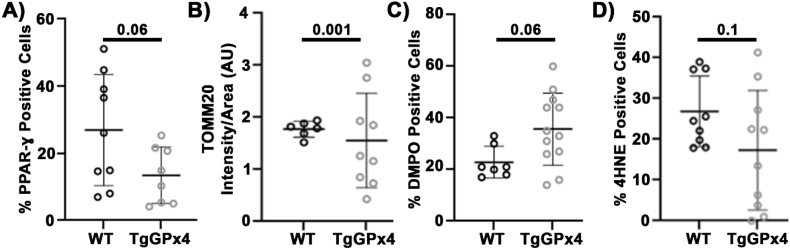
Since lipid peroxidation appears to link regulation of the redox environment and mitochondria, we wanted to examine how prolonged disruption of this pathway would affect chondrocyte redox metabolic homeostasis. TgGPx4 mice and littermates were aged to 18+ months to model chronic disruption of this redox and mitochondrial regulation in the absence of exogenous stress. We observed no indications of osteoarthritic joint degeneration in any of these aged mice. We found TgGPx4 mice demonstrated decreased PPAR-γ positivity compared to controls, Fig. 5A, suggesting that this signaling pathway may be impaired by chronic overexpression of GPx4 in the joint. We also observed the aged TgGPx4 had unusually high variance of TOMM20 intensity compared to WT, with some animals demonstrating TOMM20 staining well in excess and some well below normal controls. The coefficient of variance for the WT of 8.5% was much lower than the variance for TgGPx4 mice of 58%, Fig. 5B. This suggests a dysregulation in mitochondria in chondrocytes as a result of GPx4 overexpression. This high variance was also observed in the %positivity of DMPO (WT 27%, TgGPx4 39%), Fig. 5C and 4HNE (WT 32%, TgGPx4 85%), Fig. 5D. Statistical analyses demonstrate that these variances are significantly greater in GPx4 mice, supporting the hypothesis that chronic overexpression of GPx4 resulted in dysregulation of mitochondria and the intracellular redox environment.
Fig. 5.
Lifelong overexpression of GPx4 led to suppressed PPAR-γ, mitochondrial dysregulation, and decoupling of redox homeostasis. (A) PPAR-γ was decreased in the TgGPx4 mice. Unpaired t-test. (B) TOMM20 intensity has a significantly wider spread in the TgGPx4 compared to WT, comparison of variance f = 0.028. (C) DMPO had higher variability in the TgGPx4 than WT, comparison of variance f = 0.19. (D) 4HNE positivity was more widespread in the TgGPx4 than the WT, comparison of variance f = 0.35.
4. Discussion
Our data suggest lipid peroxidation links the intracellular redox environment of chondrocytes to mitochondrial regulation in a reciprocal relationship that is hyper-responsive acutely and can become dysregulated over time. We applied rigorous biochemical and genetic manipulations of lipid peroxidation to articular cartilage model systems both ex vivo and in vivo. These experiments showed that increasing lipid antioxidants increased chondrocyte mitochondria and increased oxidation of the intracellular redox environment; conversely, lipid prooxidants caused a consistent loss of mitochondria that led to decreases in oxidation. This paradox, that decreasing lipid peroxidation increased intracellular oxidation while increasing lipid peroxidation decreased intracellular oxidation, was consistent in each healthy chondrocyte model system utilized and was extremely responsive to very low doses of these compounds. The rapid, delicate, and potentially paradoxical responses of chondrocytes to changes in lipid peroxidation suggest the need for enrichment of redox paradigms of articular cartilage using well developed redox model systems like the TgGPx4 mouse. Because mitochondria coordinate intracellular metabolism in response to a wide variety of stresses and stimuli, lipid peroxidation-based regulation of mitochondria in chondrocytes is likely to have distinct and important effects in a wide range of physiological settings from healthy to diseased and in environs that are more oxidizing or reducing than normal.
Chondrocyte metabolism and intracellular redox environment are complex, and it is important to note some limitations of this study. First, we concentrated on mitochondrial marker TOMM20 and mitochondrial dyes for our mitochondrial measures and note that these measures have been consistent among all model systems; however, we recognize that mitochondrial membrane potential, super complex formation, and other features of mitochondrial biology can alter these signals [79,80]. Second, though the fluorescent probe BODIPY C11 can be oxidized to non-fluorescent products [81], we increased the concentration of BODIPY C11 and decreased the relative concentrations of tBOOH and VitE such that non-fluorescent products should be minimal. Third, the TgGPx4 mouse model has whole-body overexpression of GPx4, meaning other tissues of the joint also possessed elevated expression of GPx4. It is unclear whether this overexpression alters the expression or regulation of other peroxidases. Future studies would benefit from tissue specific manipulations or conditional knockouts that might allow greater control of such adaptive responses if present. Currently those models are not available or have demonstrated toxicity even in long term conditional knockouts [82].
It is important to note that the specific reactions of VitE and GPx4 are not a direct comparison. VitE requires ascorbate to reduce and recycle VitE• back to VitE. This recycling is important to decrease lipid peroxidation. In contrast, GPx4 uses glutathione for the reduction and recycling of GPx4. However, both antioxidant manipulations decrease lipid peroxidation and this was the reason we applied them here. Greater molecular specificity of redox phenomena within the lipid compartment remains elusive for many reasons. This is particularly true for the complexity and multitude of lipid oxidation products, for example as a result of the many intracellular lipoxygenases. Rigorous, careful studies of these pathways have shown how “Goldilocks” dynamics appear to be in play regarding the oxidation of lipids in cartilage [83,84], but substantial future studies are needed. Nonetheless, in chondrocytes lipid antioxidants applied here demonstrated consistent effects upon mitochondrial regulation and oxidant production through lipid peroxidation. Greater detail in the lipidomic profiling of these systems alongside rigorous redox approaches should prove highly valuable to the orthopedic research community.
Prior basic research into lipid peroxidation in chondrocytes has concentrated heavily on injury and inflammation as causal for lipid peroxidation [28,29,31,40,41]. In contrast, our study was focused on determining the homeostatic role of lipid peroxidation upon articular chondrocyte mitochondria. Several considerations were made when selecting our model to limit redox and metabolic artifacts to the greatest extent possible. First, chondrocytes are adapted to a low oxygen environment [85]; we utilized physiologically relevant 5 % oxygen cultures that offer a lower oxygen tension than some models but should largely correspond to much of the research in the orthopedic community. Second, we have avoided use of monolayer culture of primary chondrocytes, which can alter respiratory function, upregulate mitochondria, which could alter metabolism and the redox environment [86,87]. Third, prior research has often used doses of redox-related compounds in excess of physiologic ranges for modeling purposes [28,30,88]. For this study, we chose minimal antioxidant doses determined via direct dose response in primary tissue to evaluate the effect of subtle changes to the redox environment. In large osteochondral explants, the magnitude of chondrocytes’ response to nM doses of lipid antioxidants and prooxidants was surprising to us and we believe reflects a special sensitivity of chondrocytes to these agents.
Some of our results may help explain why examination of lipid peroxidation has produced disconnects between in vitro and in vivo models [21,45,50,83,89,90]. Our findings suggest a regulatory paradox likely to confound a large variety of redox-focused inquiries both in vitro and in vivo. One illustrative example is iron chelation, which decreases the amount of redox active iron. Redox active iron promotes lipid peroxidation and therefore iron chelation would be hypothesized to decrease the formation of 4HNE on proteins based upon most published applications of the technique. In the Dunkin-Hartley guinea pig model, iron chelation is protective against OA, yet chelation treatment increased cartilage 4HNE [91] despite 4HNE's common association with injury [24,28,30]. Effectively, iron chelation behaved similarly to GPx4 overexpression in young mice, increasing 4HNE production by preserving or augmenting the oxidizing components of chondrocytes, likely mitochondria. We propose that these physiological effects in the Dunkin-Hartley guinea pig outline a delicate and responsive iron biology in chondrocytes to complement the lipid redox biology we have shown here.
This relationship may explain why certain redox focused approaches have struggled to translate clinically, particularly clinical trials of VitE [[47], [48], [49],53]. Our results show that VitE increases chondrocyte mitochondrial markers and alters the redox environment in such a way that chronic oxidative stress could be increased by prolonged treatment with this radical scavenger. Lifelong elevation of GPx4 resulted in severely dysregulated chondrocyte mitochondria and decreased PPAR-γ, a pathway typically associated with protection against OA [89]. This may link to increased radiographic OA in patients with the highest levels of VitE [48], but additional studies are needed. Though we were able to replicate an antioxidant effect of VitE in stressed conditions, that benefit was confined entirely to tissue that received a coincident, mechanical injury. When considering the daily activities of an individual over a lifetime of acquiring OA, future prevention strategies may benefit from incorporating more refined understanding of healthy chondrocyte redox paradigms.
To attempt to describe chronic dysfunction of intraarticular lipid peroxidation in the absence of overt injury, we aged TgGPx4 mice and WT littermates to 18+ months of age. Our results support a role for lipid peroxidation-based regulation of chondrocyte mitochondria during aging, but the C57Bl/6J background of the TgGPx4 strain is not well suited to developing age-induced OA and no indications of joint degeneration were seen in these mice. Nonetheless, it appears this regulatory mechanism has a relationship to PPAR-γ that can become deranged during the aging process, and this may be an important clue to redox pathology during chronic joint degeneration. Lipid peroxidation is known to be important for regulation of mitochondria by PPAR-γ during aging [45,89] and many studies have demonstrated PPAR-γ′s importance to cartilage health during injury [92].
5. Conclusions
In conclusion, our findings support the hypothesis that lipid peroxidation links the chondrocyte redox environment to mitochondrial regulation during normal, healthy function. Prior literature suggests chondrocyte intracellular thiols are maintained at a relatively oxidized state such that fluctuations are more likely to be observed in lipid peroxidation. Chondrocytes were demonstrated to be highly responsive to even modest lipid antioxidant and prooxidant stimuli. These responses occur well within the timeframe of most benchtop experimentation and include potent regulation of mitochondria and large, sometimes paradoxical swings in the intracellular redox environment. This reciprocal relationship between mitochondria and lipid oxidants/antioxidants appears critical to maintaining normal joint redox metabolism during aging.
Funding sources
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin (AR070914) as well as internal funding from the departments of Radiation Oncology and Orthopedics and Rehabilitation at the University of Iowa, Carver College of Medicine.
CRediT authorship contribution statement
Madeline R. Hines: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Piedad C. Gomez-Contreras: Writing – review & editing, Methodology, Formal analysis, Data curation. Suryamin Liman: Writing – review & editing, Methodology, Formal analysis. Alexandria M. Wilson: Writing – review & editing, Formal analysis. Kevin J. Lu: Writing – review & editing, Formal analysis. Jaycie A. O'Neill: Writing – review & editing, Formal analysis. Jacob S. Fisher: Writing – review & editing, Formal analysis. Douglas C. Fredericks: Writing – review & editing, Supervision, Methodology, Conceptualization. Brett A. Wagner: Writing – review & editing, Methodology, Investigation, Conceptualization. Garry R. Buettner: Writing – review & editing, Supervision, Methodology, Conceptualization. Holly Van Remmen: Writing – review & editing, Methodology, Conceptualization. Mitchell C. Coleman: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
None.
Acknowledgements
In addition to our funders at NIAMS, we would like to acknowledge the gift of the anti-DMPO antibody from Ronald Mason, PhD, National Institute of Environmental Health Sciences. The University of Iowa Orthopedics Department for ongoing support, the Bone Healing Laboratory, and the Orthopedic Histology Service Center for their outstanding technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103306.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Brouillette M.J., et al. Strain-dependent oxidant release in articular cartilage originates from mitochondria. Biomech. Model. Mechanobiol. 2014;13(3):565–572. doi: 10.1007/s10237-013-0518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman M.C., et al. Injurious loading of articular cartilage compromises chondrocyte respiratory function. Arthritis Rheumatol. 2016;68(3):662–671. doi: 10.1002/art.39460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin J.A., et al. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91(8):1890–1897. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetz J.E., et al. Time-dependent loss of mitochondrial function precedes progressive histologic cartilage degeneration in a rabbit meniscal destabilization model. J. Orthop. Res. 2017;35(3):590–599. doi: 10.1002/jor.23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Contreras P.C., et al. Intersections between mitochondrial metabolism and redox biology mediate posttraumatic osteoarthritis. Curr. Rheumatol. Rep. 2021;23(5):32. doi: 10.1007/s11926-021-00994-z. [DOI] [PubMed] [Google Scholar]
- 6.Coleman M.C., et al. Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Sci. Transl. Med. 2018;10(427) doi: 10.1126/scitranslmed.aan5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari M.Y., et al. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26(8):1087–1097. doi: 10.1016/j.joca.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansari M.Y., Novak K., Haqqi T.M. ERK1/2-mediated activation of DRP1 regulates mitochondrial dynamics and apoptosis in chondrocytes. Osteoarthritis Cartilage. 2022;30(2):315–328. doi: 10.1016/j.joca.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarsour E.H., Kalen A.L., Goswami P.C. Manganese superoxide dismutase regulates a redox cycle within the cell cycle. Antioxidants Redox Signal. 2014;20(10):1618–1627. doi: 10.1089/ars.2013.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon S.G., Goswami P.C. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26(8):1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 11.Forman H.J., Ursini F., Maiorino M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones D.P. Redox theory of aging. Redox Biol. 2015;5:71–79. doi: 10.1016/j.redox.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aruoma O.I., Kaur H., Halliwell B. Oxygen free radicals and human diseases. J. Roy. Soc. Health. 1991;111(5):172–177. doi: 10.1177/146642409111100506. [DOI] [PubMed] [Google Scholar]
- 14.Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27(9–10):916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 15.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 16.O'Malley Y., et al. Oxidative stress and impaired insulin secretion in cystic fibrosis pig pancreas. Adv Redox Res. 2022;5 doi: 10.1016/j.arres.2022.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison J.P., et al. Thiol supplementation in aged animals alters antioxidant enzyme activity after heat stress. J. Appl. Physiol. 2005;99(6):2271–2277. doi: 10.1152/japplphysiol.00412.2005. (1985) [DOI] [PubMed] [Google Scholar]
- 18.Buettner G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993;300(2):535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 19.Bhatti F.U., et al. Vitamin E protects chondrocytes against hydrogen peroxide-induced oxidative stress in vitro. Inflamm. Res. 2013;62(8):781–789. doi: 10.1007/s00011-013-0635-y. [DOI] [PubMed] [Google Scholar]
- 20.Beecher B.R., et al. Antioxidants block cyclic loading induced chondrocyte death. Iowa Orthop. J. 2007;27:1–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Tiku M.L., Gupta S., Deshmukh D.R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic. Res. 1999;30(5):395–405. doi: 10.1080/10715769900300431. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson M., Jones B.S. Serum and synovial fluid proteins in arthritis. Ann. Rheum. Dis. 1962;21:51–58. doi: 10.1136/ard.21.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings N.A., Nordby G.L. Measurement of synovial fluid pH in normal and arthritic knees. Arthritis Rheum. 1966;9(1):47–56. doi: 10.1002/art.1780090106. [DOI] [PubMed] [Google Scholar]
- 24.Selley M.L., et al. Occurrence of (E)-4-hydroxy-2-nonenal in plasma and synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 1992;51(4):481–484. doi: 10.1136/ard.51.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNearney T., et al. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J. Rheumatol. 2000;27(3):739–745. [PMC free article] [PubMed] [Google Scholar]
- 26.Angthong C., et al. Can levels of antioxidants in synovial fluid predict the severity of primary knee osteoarthritis: a preliminary study. SpringerPlus. 2013;2:652. doi: 10.1186/2193-1801-2-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydogan N.H., et al. The effect of arthroscopic surgery and intraarticular drug injection to the antioxidation system and lipid peroxidation at osteoarthritis of knee. Saudi Med. J. 2008;29(3):397–402. [PubMed] [Google Scholar]
- 28.Morquette B., et al. Production of lipid peroxidation products in osteoarthritic tissues: new evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis Rheum. 2006;54(1):271–281. doi: 10.1002/art.21559. [DOI] [PubMed] [Google Scholar]
- 29.Ostalowska A., et al. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthritis Cartilage. 2006;14(2):139–145. doi: 10.1016/j.joca.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Shi Q., et al. New evidence implicating 4-hydroxynonenal in the pathogenesis of osteoarthritis in vivo. Arthritis Rheumatol. 2014;66(9):2461–2471. doi: 10.1002/art.38704. [DOI] [PubMed] [Google Scholar]
- 31.Sutipornpalangkul W., et al. Lipid peroxidation, glutathione, vitamin E, and antioxidant enzymes in synovial fluid from patients with osteoarthritis. Int J Rheum Dis. 2009;12(4):324–328. doi: 10.1111/j.1756-185X.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 32.Shah R., Raska K., Jr., Tiku M.L. The presence of molecular markers of in vivo lipid peroxidation in osteoarthritic cartilage: a pathogenic role in osteoarthritis. Arthritis Rheum. 2005;52(9):2799–2807. doi: 10.1002/art.21239. [DOI] [PubMed] [Google Scholar]
- 33.Vaillancourt F., et al. Differential regulation of cyclooxygenase-2 and inducible nitric oxide synthase by 4-hydroxynonenal in human osteoarthritic chondrocytes through ATF-2/CREB-1 transactivation and concomitant inhibition of NF-kappaB signaling cascade. J. Cell. Biochem. 2007;100(5):1217–1231. doi: 10.1002/jcb.21110. [DOI] [PubMed] [Google Scholar]
- 34.Chen K., et al. Increased 15-lipoxygenase-1 expression in chondrocytes contributes to the pathogenesis of osteoarthritis. Cell Death Dis. 2017;8(10):e3109. doi: 10.1038/cddis.2017.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins J.A., et al. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signaling in aging chondrocytes. J. Biol. Chem. 2016;291(13):6641–6654. doi: 10.1074/jbc.M115.693523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loeser R.F., et al. Methylation of the OP-1 promoter: potential role in the age-related decline in OP-1 expression in cartilage. Osteoarthritis Cartilage. 2009;17(4):513–517. doi: 10.1016/j.joca.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loeser R.F., et al. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for alpha1beta1 and alpha2beta1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis Cartilage. 2000;8(2):96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- 38.Taylor E.L., et al. Age and oxidative stress regulate Nrf 2 homeostasis in human articular chondrocytes. Osteoarthritis Cartilage. 2023;31(9):1214–1223. doi: 10.1016/j.joca.2023.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abusarah J., et al. An overview of the role of lipid peroxidation-derived 4-hydroxynonenal in osteoarthritis. Inflamm. Res. 2017;66(8):637–651. doi: 10.1007/s00011-017-1044-4. [DOI] [PubMed] [Google Scholar]
- 40.Chrubasik S. Vitamin E for rheumatoid arthritis or osteoarthritis: low evidence of effectiveness. Z. Rheumatol. 2003;62(5):491. doi: 10.1007/s00393-003-0553-4. [DOI] [PubMed] [Google Scholar]
- 41.Surapaneni K.M., Venkataramana G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J. Med. Sci. 2007;61(1):9–14. [PubMed] [Google Scholar]
- 42.Itoh T., et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008;15(9):924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corona J.C., Duchen M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016;100:153–163. doi: 10.1016/j.freeradbiomed.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasheghani F., et al. Adult cartilage-specific peroxisome proliferator-activated receptor gamma knockout mice exhibit the spontaneous osteoarthritis phenotype. Am. J. Pathol. 2013;182(4):1099–1106. doi: 10.1016/j.ajpath.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Vasheghani F., et al. PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann. Rheum. Dis. 2015;74(3):569–578. doi: 10.1136/annrheumdis-2014-205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi T., et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, reduces the progression of experimental osteoarthritis in Guinea pigs. Arthritis Rheum. 2005;52(2):479–487. doi: 10.1002/art.20792. [DOI] [PubMed] [Google Scholar]
- 47.Canter P.H., Wider B., Ernst E. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology. 2007;46(8):1223–1233. doi: 10.1093/rheumatology/kem116. [DOI] [PubMed] [Google Scholar]
- 48.Chaganti R.K., et al. High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190–196. doi: 10.1016/j.joca.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wluka A.E., et al. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. J. Rheumatol. 2002;29(12):2585–2591. [PubMed] [Google Scholar]
- 50.Tiku M.L., Shah R., Allison G.T. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J. Biol. Chem. 2000;275(26):20069–20076. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 51.Tiku M.L., et al. Malondialdehyde oxidation of cartilage collagen by chondrocytes. Osteoarthritis Cartilage. 2003;11(3):159–166. doi: 10.1016/s1063-4584(02)00348-5. [DOI] [PubMed] [Google Scholar]
- 52.Vaillancourt F., et al. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res. Ther. 2008;10(5):R107. doi: 10.1186/ar2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chin K.Y., Ima-Nirwana S. The role of vitamin E in preventing and treating osteoarthritis - a review of the current evidence. Front. Pharmacol. 2018;9:946. doi: 10.3389/fphar.2018.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ran Q., et al. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J. Biol. Chem. 2004;279(53):55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 55.Heller A., Ulstrup J. Detlev müller's discovery of glucose oxidase in 1925. Anal. Chem. 2021;93(18):7148–7149. doi: 10.1021/acs.analchem.1c01191. [DOI] [PubMed] [Google Scholar]
- 56.Thornalley P.J., Trotta R.J., Stern A. Free radical involvement in the oxidative phenomena induced by tert-butyl hydroperoxide in erythrocytes. Biochim. Biophys. Acta. 1983;759(1–2):16–22. doi: 10.1016/0304-4165(83)90183-6. [DOI] [PubMed] [Google Scholar]
- 57.Hunziker E.B., Graber W. Differential extraction of proteoglycans from cartilage tissue matrix compartments in isotonic buffer salt solutions and commercial tissue-culture media. J. Histochem. Cytochem. 1986;34(9):1149–1153. doi: 10.1177/34.9.2426342. [DOI] [PubMed] [Google Scholar]
- 58.Hunziker E.B., Herrmann W., Schenk R.K. Ruthenium hexammine trichloride (RHT)-mediated interaction between plasmalemmal components and pericellular matrix proteoglycans is responsible for the preservation of chondrocytic plasma membranes in situ during cartilage fixation. J. Histochem. Cytochem. 1983;31(6):717–727. doi: 10.1177/31.6.6341460. [DOI] [PubMed] [Google Scholar]
- 59.Hines M.R., et al. Extracellular biomolecular free radical formation during injury. Free Radic. Biol. Med. 2022;188:175–184. doi: 10.1016/j.freeradbiomed.2022.06.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clutton G., et al. A reproducible, objective method using MitoTracker® fluorescent dyes to assess mitochondrial mass in T cells by flow cytometry. Cytometry. 2019;95(4):450–456. doi: 10.1002/cyto.a.23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drummen G.P., et al. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002;33(4):473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 62.Liang H., et al. Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria. Free Radic. Biol. Med. 2009;47(3):312–320. doi: 10.1016/j.freeradbiomed.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khoo N.K., et al. Obesity-induced tissue free radical generation: an in vivo immuno-spin trapping study. Free Radic. Biol. Med. 2012;52(11–12):2312–2319. doi: 10.1016/j.freeradbiomed.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khoo N.K., et al. In vivo immuno-spin trapping: imaging the footprints of oxidative stress. Curr Protoc Cytom. 2015;74:12.42.1–12.4211. doi: 10.1002/0471142956.cy1242s74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mason R.P. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic. Biol. Med. 2004;36(10):1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 66.Mason R.P. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol. 2016;8:422–429. doi: 10.1016/j.redox.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Summers F.A., Mason R.P., Ehrenshaft M. Development of immunoblotting techniques for DNA radical detection. Free Radic. Biol. Med. 2013;56:64–71. doi: 10.1016/j.freeradbiomed.2012.10.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mason R.P., Ganini D. Immuno-spin trapping of macromolecules free radicals in vitro and in vivo - one stop shopping for free radical detection. Free Radic. Biol. Med. 2019;131:318–331. doi: 10.1016/j.freeradbiomed.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Cox A.G., Winterbourn C.C., Hampton M.B. Measuring the redox state of cellular peroxiredoxins by immunoblotting. Methods Enzymol. 2010;474:51–66. doi: 10.1016/S0076-6879(10)74004-0. [DOI] [PubMed] [Google Scholar]
- 70.Del Dotto V., et al. Eight human OPA1 isoforms, long and short: what are they for? Biochim. Biophys. Acta Bioenerg. 2018;1859(4):263–269. doi: 10.1016/j.bbabio.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 71.von der Malsburg A., et al. Structural mechanism of mitochondrial membrane remodelling by human OPA1. Nature. 2023;620(7976):1101–1108. doi: 10.1038/s41586-023-06441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Dotto V., et al. OPA1 isoforms in the hierarchical organization of mitochondrial functions. Cell Rep. 2017;19(12):2557–2571. doi: 10.1016/j.celrep.2017.05.073. [DOI] [PubMed] [Google Scholar]
- 73.Song Z., et al. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 2007;178(5):749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishihara N., et al. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25(13):2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wood Z.A., Poole L.B., Karplus P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300(5619):650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 76.Gomez-Mejiba S.E., et al. Immuno-spin trapping from biochemistry to medicine: advances, challenges, and pitfalls. Focus on protein-centered radicals. Biochim. Biophys. Acta. 2014;1840(2):722–729. doi: 10.1016/j.bbagen.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coleman M.C., et al. Differential effects of superoxide dismutase mimetics after mechanical overload of articular cartilage. Antioxidants. 2017;6(4) doi: 10.3390/antiox6040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodwin W., et al. Rotenone prevents impact-induced chondrocyte death. J. Orthop. Res. 2010;28(8):1057–1063. doi: 10.1002/jor.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polster B.M., et al. Use of potentiometric fluorophores in the measurement of mitochondrial reactive oxygen species. Methods Enzymol. 2014;547:225–250. doi: 10.1016/B978-0-12-801415-8.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neikirk K., et al. MitoTracker: a useful tool in need of better alternatives. Eur. J. Cell Biol. 2023;102(4) doi: 10.1016/j.ejcb.2023.151371. [DOI] [PubMed] [Google Scholar]
- 81.MacDonald M.L., Murray I.V., Axelsen P.H. Mass spectrometric analysis demonstrates that BODIPY 581/591 C11 overestimates and inhibits oxidative lipid damage. Free Radic. Biol. Med. 2007;42(9):1392–1397. doi: 10.1016/j.freeradbiomed.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 82.Yoo S.E., et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012;52(9):1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chabane N., et al. Human articular chondrocytes express 15-lipoxygenase-1 and -2: potential role in osteoarthritis. Arthritis Res. Ther. 2009;11(2):R44. doi: 10.1186/ar2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Habouri L., et al. Deletion of 12/15-lipoxygenase accelerates the development of aging-associated and instability-induced osteoarthritis. Osteoarthritis Cartilage. 2017;25(10):1719–1728. doi: 10.1016/j.joca.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Zhou S., Cui Z., Urban J.P. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50(12):3915–3924. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 86.Mignotte F., et al. Mitochondrial biogenesis in rabbit articular chondrocytes transferred to culture. Biol. Cell. 1991;71(1–2):67–72. doi: 10.1016/0248-4900(91)90052-o. [DOI] [PubMed] [Google Scholar]
- 87.Boubriak O.A., Brooks J.T., Urban J.P. Cytochrome c oxidase levels in chondrocytes during monolayer expansion and after return to three dimensional culture. Osteoarthritis Cartilage. 2009;17(8):1084–1092. doi: 10.1016/j.joca.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Zayed N., et al. Increased expression of lipocalin-type prostaglandin D2 synthase in osteoarthritic cartilage. Arthritis Res. Ther. 2008;10(6):R146. doi: 10.1186/ar2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fahmi H., et al. Peroxisome proliferator-activated receptor gamma in osteoarthritis. Mod. Rheumatol. 2011;21(1):1–9. doi: 10.1007/s10165-010-0347-x. [DOI] [PubMed] [Google Scholar]
- 90.Nebbaki S.S., et al. Expression of peroxisome proliferator-activated receptors α, β, γ, and H- and L-prostaglandin D synthase during osteoarthritis in the spontaneous hartley Guinea pig and experimental dog models. J. Rheumatol. 2013;40(6):877–890. doi: 10.3899/jrheum.120738. [DOI] [PubMed] [Google Scholar]
- 91.Burton L.H., et al. Systemic administration of a pharmacologic iron chelator reduces cartilage lesion development in the Dunkin-Hartley model of primary osteoarthritis. Free Radic. Biol. Med. 2022;179:47–58. doi: 10.1016/j.freeradbiomed.2021.12.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu X., et al. PPARγ preservation via promoter demethylation alleviates osteoarthritis in mice. Ann. Rheum. Dis. 2019;78(10):1420–1429. doi: 10.1136/annrheumdis-2018-214940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.