Abstract
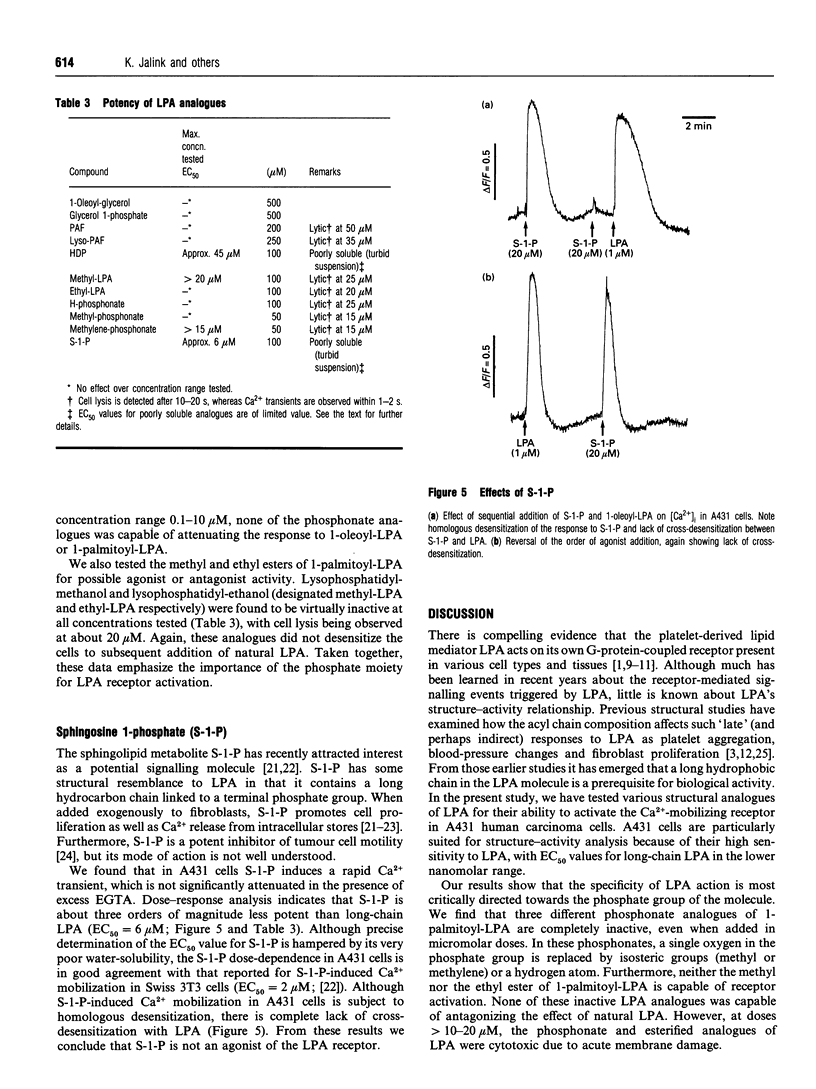
Lysophosphatidic acid (LPA; 1-acyl-sn-glycero-3-phosphate) is a platelet-derived lipid mediator that activates its own G-protein-coupled receptor to trigger phospholipase C-mediated Ca2+ mobilization and other effector pathways in numerous cell types. In this study we have examined the structural features of LPA that are important for activation of the Ca(2+)-mobilizing receptor in human A431 carcinoma cells, which show an EC50 for oleoyl-LPA as low as 0.2 nM. When the acyl chain at the sn-1 position is altered, the rank order of potency is oleoyl-LPA > arachidonoyl-LPA > linolenoyl-LPA > linoleoyl-LPA > stearoyl-LPA = palmitoyl-LPA > myristoyl-LPA. The shorter-chain species, lauroyl- and decanoyl-LPA, show little or no activity. Ether-linked LPA (1-O-hexadecyl-sn-glycero-3-phosphate) is somewhat less potent than the corresponding ester-linked LPA; its stereoisomer is about equally active. Deletion of the glycerol backbone causes a 1000-fold decrease in potency. Replacement of the phosphate group in palmitoyl-LPA by a hydrogen- or methyl-phosphonate moiety results in complete loss of activity. A phosphonate analogue with a methylene group replacing the oxygen at sn-3 has strongly decreased activity. All three phosphonate analogues induce cell lysis at doses > 15 microM. Similarly, the methyl and ethyl esters of palmitoyl-LPA are virtually inactive and become cytotoxic at micromolar doses. None of the LPA analogues tested has antagonist activity. Sphingosine 1-phosphate, a putative messenger with some structural similarities to LPA, elicits a transient rise in intracellular [Ca2+] only at micromolar doses; however, cross-desensitization experiments indicate that sphingosine 1-phosphate does not act through the LPA receptor. The results indicate that, although many features of the LPA structure are important for optimal activity, the phosphate group is most critical, suggesting that this moiety is directly involved in receptor activation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coughlin S. R. Expanding horizons for receptors coupled to G proteins: diversity and disease. Curr Opin Cell Biol. 1994 Apr;6(2):191–197. doi: 10.1016/0955-0674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Durieux M. E., Carlisle S. J., Salafranca M. N., Lynch K. R. Responses to sphingosine-1-phosphate in X. laevis oocytes: similarities with lysophosphatidic acid signaling. Am J Physiol. 1993 May;264(5 Pt 1):C1360–C1364. doi: 10.1152/ajpcell.1993.264.5.C1360. [DOI] [PubMed] [Google Scholar]
- Eichholtz T., Jalink K., Fahrenfort I., Moolenaar W. H. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993 May 1;291(Pt 3):677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta. 1989 Feb 20;1001(3):282–285. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- Krabak M. J., Hui S. W. The mitogenic activities of phosphatidate are acyl-chain-length dependent and calcium independent in C3H/10T1/2 cells. Cell Regul. 1991 Jan;2(1):57–64. doi: 10.1091/mbc.2.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H. LPA: a novel lipid mediator with diverse biological actions. Trends Cell Biol. 1994 Jun;4(6):213–219. doi: 10.1016/0962-8924(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Sadahira Y., Ruan F., Hakomori S., Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9686–9690. doi: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono S., Kawamoto K., Yoshida N., Kondo T., Inagami T. Neurotransmitter release from lysophosphatidic acid stimulated PC12 cells: involvement of lysophosphatidic acid receptors. Biochem Biophys Res Commun. 1993 Jun 15;193(2):667–673. doi: 10.1006/bbrc.1993.1676. [DOI] [PubMed] [Google Scholar]
- Simon M. F., Chap H., Douste-Blazy L. Human platelet aggregation induced by 1-alkyl-lysophosphatidic acid and its analogs: a new group of phospholipid mediators? Biochem Biophys Res Commun. 1982 Oct 29;108(4):1743–1750. doi: 10.1016/s0006-291x(82)80113-7. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Tokumura A., Gregory L., Nouchi T., Weintraub S. T., Hanahan D. J. Biochemical characterization of the interaction of lipid phosphoric acids with human platelets: comparison with platelet activating factor. Arch Biochem Biophys. 1994 Jun;311(2):358–368. doi: 10.1006/abbi.1994.1249. [DOI] [PubMed] [Google Scholar]
- Thomson F. J., Perkins L., Ahern D., Clark M. Identification and characterization of a lysophosphatidic acid receptor. Mol Pharmacol. 1994 Apr;45(4):718–723. [PubMed] [Google Scholar]
- Tigyi G., Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992 Oct 25;267(30):21360–21367. [PubMed] [Google Scholar]
- Tilly B. C., van Paridon P. A., Verlaan I., Wirtz K. W., de Laat S. W., Moolenaar W. H. Inositol phosphate metabolism in bradykinin-stimulated human A431 carcinoma cells. Relationship to calcium signalling. Biochem J. 1987 May 15;244(1):129–135. doi: 10.1042/bj2440129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly B. C., van Paridon P. A., Verlaan I., de Laat S. W., Moolenaar W. H. Epidermal-growth-factor-induced formation of inositol phosphates in human A431 cells. Differences from the effect of bradykinin. Biochem J. 1988 Jun 15;252(3):857–863. doi: 10.1042/bj2520857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumura A., Fukuzawa K., Isobe J., Tsukatani H. Lysophosphatidic acid-induced aggregation of human and feline platelets: structure-activity relationship. Biochem Biophys Res Commun. 1981 Mar 31;99(2):391–398. doi: 10.1016/0006-291x(81)91758-7. [DOI] [PubMed] [Google Scholar]
- Tokumura A., Fukuzawa K., Tsukatani H. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids. 1978 Aug;13(8):572–574. doi: 10.1007/BF02533598. [DOI] [PubMed] [Google Scholar]
- Tokumura A., Harada K., Fukuzawa K., Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta. 1986 Jan 3;875(1):31–38. [PubMed] [Google Scholar]
- Tokumura A., Kume T., Fukuzawa K., Tsukatani H. Cardiovascular effects of lysophosphatidic acid and its structural analogs in rats. J Pharmacol Exp Ther. 1981 Oct;219(1):219–224. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Foglesong R. J., Bell R. M. A facile enzymatic synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. J Lipid Res. 1989 Apr;30(4):611–616. [PubMed] [Google Scholar]
- Zhang H., Desai N. N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991 Jul;114(1):155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bend R. L., Brunner J., Jalink K., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. Identification of a putative membrane receptor for the bioactive phospholipid, lysophosphatidic acid. EMBO J. 1992 Jul;11(7):2495–2501. doi: 10.1002/j.1460-2075.1992.tb05314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


