Abstract
Purpose
Chemical dissolution of nickel-titanium (NiTi) files involves the application of a fluoride solution in direct contact with a damaged instrument, whereas electrochemical dissolution involves the application of an electrical current to the electrolyte, which accelerates fragment dissolution. This study aimed to determine the hardness and concentration of calcium and phosphorus (Ca and P) ions in dentinal walls following chemical and electrochemical dissolution of fractured ProTaper F2 files with a novel chemical solution.
Materials & Methods: Thirty human maxillary first molar palatal roots with fractured ProTaper F2 files in the middle third (length, 2.5 mm were divided into three groups according to the treatment techniques used with a novel solution (NaF 12 g/L + NaCl2 60 g/L + MgCl2 60 g/L + CaCl2 60 g/L) at pH 5: Group 1: distilled water (control group), Group 2: electrochemical dissolution, and Group 3: chemical dissolution using the novel solution. The novel solution was placed for 10 min using an electrochemical technique, and for 30 min in contact with the separated instrument in the chemical group. The Vickers microhardness test was performed in three areas: at 3, 6, and 9 mm from the apex, and an energy-dispersive X-ray test for both Ca and P ions was performed. The analysis of variance (ANOVA) and Tukey's tests were used for statistical analysis.
Results
According to the one-way ANOVA analysis, no difference was observed between the tested approaches (P > 0.05) in the three areas evaluated (3, 6, and 9 mm), with no difference in the Ca/P ratio between the tested groups.
Conclusion
Compared to the control group, the use of chemical and electrochemical dissolution methods with the novel solution did not affect dentin hardness or dentinal structure in terms of the Ca/P ratio, thereby indicating promising results while saving time.
Keywords: Microhardness, Chemical, Electrochemical, Dissolution, Fractured NiTi files
Highlights
-
•
A novel fluoride solution was used for nickel-titanium (NiTi) file dissolution.
-
•
The new solution did not alter the dentinal structure.
-
•
Electrochemical and chemical processes exhibited similarities.
-
•
The solutions did not affect the Ca/P ratio in dentin.
-
•
Our study demonstrated promising results for NiTi file management.
1. Introduction
In the field of endodontics, the management of fractured nickel-titanium (NiTi) files inside a root canal can be challenging, especially in cases of persisting previous infection or inadequate cleaning and disinfection [1,2]. Prescribed protocols for the removal of a fractured file from the root canal may result in the development of complications and iatrogenic issues, such as root weakening and perforation [[3], [4], [5]]. Thus, research has focussed on conservative techniques for the removal of the fractured endodontic files, such as electrochemical disintegration [6,7]. Fluoride-based solutions are commonly used to dissolve the electrochemical endodontic files. This approach entails active partial or total dissolution of the separated instrument by electrochemical dissolution using two electrodes (cathode and anode) in an electrolyte. Upon electric potential supply between the electrodes, the electrons migrate, releasing metallic ions into the solution [8]. Various solutions of various concentrations have been proposed [7]. In addition to the low pH (5.0) of these experimental solutions, which is a major element favouring corrosion and permitting exposure to the solution, weakening the NiTi alloy, the detrimental effect of the solution on the dentinal structure is very concerning, which might restrict its usage [8,9]. Dentin structure, such as the quantity of dentin and calcium/phosphorus (Ca/P) ratio, has been studied following the use of chemical solutions in endodontic procedures. In addition, Ni and Ti ions can be impregnated into dentinal tubules via chemical processes [8,9].
The use of fluoride-based solutions has time limitations, as they require prolonged clinical time [10] and more ions to bind to the Ni and Ti ions. Therefore, the novel solution used in this study had a new formula of NaF 12 g/L + NaCl2 60 g/L + MgCl2 60 g/L + CaCl2 60 g/L with a pH of 5 and additional ions to decrease the time required for the electrochemical dissolution of the fractured file to ensure clinical predictability with the construction of a new device, which can be used clinically for electrochemical dissolution without the need for dentin removal or use of other sensitive techniques; therefore, their effect on the hardness and calcium and phosphorus (Ca and P) ions of the dentinal following both techniques with the use of a novel chemical solution should be tested. The null hypothesis was that the use of this novel solution can affect the hardness and chemical composition of dentin when used with electrochemical and chemical dissolution techniques to manage fractured NiTi files.
2. Materials and methods
This study was approved by the ethics committee of the University of Baghdad (approval number: 637) and was conducted in accordance with the Declaration of Helsinki for both chemical and electrochemical dissolution processes with fractured ProTaper NiTi instruments (F2) (Dentsply, Switzerland) inside the canal. This study included 30 palatal roots of maxillary first molars.
2.1. Grouping
Thirty palatal roots of the maxillary first molars were used in this study and divided into three groups according to the treatment technique using the novel solution (n = 10):
Group 1: Samples treated with normal saline (control group).
Group 2: Samples treated with electrochemical dissolution.
Group 3: Samples treated with chemical dissolution.
2.2. Intracanal fragment dissolution
The sample size was calculated using a 30 percent effect in volume variation, a 25 percent standard deviation response, an alpha of 0.05, a power of 0.80, and a power analysis. Considering these assumptions, the sample size for each group was set at 10.
2.3. Sample selection and preparation
The palatal roots of the maxillary molars (n = 10) in each group used in this study met the following inclusion criteria: single canals with completely formed apices, no calcification or resorption, and roots of similar length. The exclusion criteria included endodontically treated teeth and teeth with cracks [11]. The teeth were stored in 10 % formalin solution until needed. To eliminate any remaining formalin, the teeth were washed under running water [12]. The teeth were initially radiographed, and the pulp chamber was opened with continuous irrigation using diamond burs. A size 08 K-file (Dentsply Sirona, Ballaigues, Switzerland) was passively placed inside the palatal root canal up to the apical foramen to assess foramen patency and measure the root canal length, which varied from 20 to 22 mm [13]. To weaken the F2 instrument, a diamond bur was used 2.5 mm from the instrument tip [14].
Each instrument was inserted with constant apical pressure into the palatal root canal and spun at 300 rpm using an electric motor (X-smart, USA) until the tool fractured and the fragment was inserted into the root canal (Fig. 1 A) [10]. The position of each piece in the middle third of the root canal was verified using radiographs of the teeth following fracture. An experienced endodontist used a size 08 K file to determine whether the file could bypass the fractured file. To validate the site of the fragment in the root's middle third, a post-fracture radiograph was taken [10].
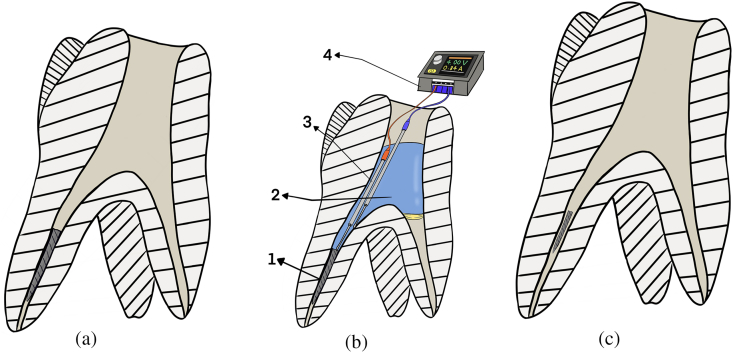
Fig. 1.
Digital drawing for the electrochemical technique used in this study: A: maxillary 1st molar with fractured file in the middle third of the palatal root. B: Electrochemical technique 1. Tooth with fractured nickel-titanium (NiTi) files in the middle third, 2. The canal space is filled with fluid, 3. two electrodes inside the root canal and in direct contact with the fractured file. 4. Specially constructed electrochemical dissolution device. C. Tooth after electrochemical technique; the fractured file is smaller in size and can be easily bypassed.
The teeth were pressed into a rubber stopper within a hole pre-drilled in a vial to avoid direct manipulation. A rubber dam sheet was placed over the roots of the vial's root [12]. To form a reservoir for the solution, each tooth's dental crown was attached to an acrylic microcell, which had an internal volume of 516 mL and an internal diameter of 9.12 mm3 during the dissolving procedure [10].
This study employed 600 mL of a novel solution (NaF 12 g/L + NaCl2 60 g/L + MgCl2 60 g/L + CaCl2 60 g/L) with pH 5.0 based on the formulation reported in a previous pilot study. The solution was placed in a microcell for both the chemical and electrochemical techniques. In the electrochemical technique, the intracanal fragment was touched with the tip of a 0.2 mm platinum wire (China) [10], which was confirmed by radiography. The working electrode (WE) was composed of a platinum wire. The reference (RE) and counter (CE) electrodes were connected by a second gold wire, 0.5 mm diameter). Each wire was submerged in the acrylic microcell solution and connected to +4.0 V potential provided by a special electrical device (Fig. 1B), which was connected to an ammeter measurement gauge that can register the anodic current reaction during the test, as shown in Fig. 1C, for 10 min to cause partial dissolution of the NiTi file to easily bypass it, based on the spectrophotometer (Hamilton, Japan) test for dissolution of both Ni and Ti in relation to time.
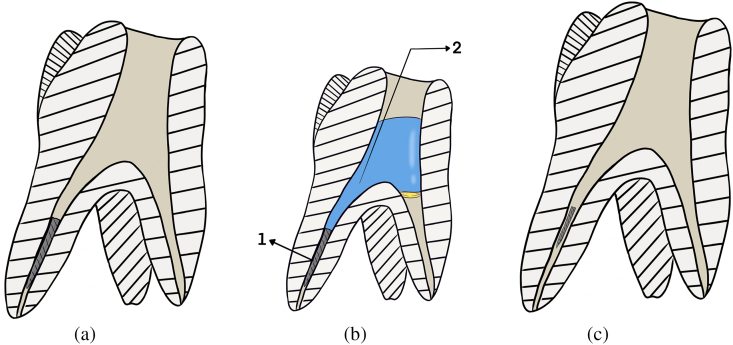
During the chemical procedure (Fig. 2A), the chemical solution was placed inside the canal for 30 min (Fig. 2B), dissolution decreased with time with increased electrical charge generated during the dissolution procedure. Therefore, new chemical solution was added every 15 min. This technique required 30 min based on a spectrophotometer test for the dissolution of both Ni and Ti in relation to time. The same endodontist passively inserted a size 08 K-file into the root canal to check whether the fragment could be bypassed (Fig. 2C).
Fig. 2.
Digital drawing of the chemical dissolution technique used in this study. A: maxillary 1st molar with fractured file in the middle third of the palatal root. B: Chemical technique where 1. Tooth with fractured NiTi files in the middle third, 2. The canal space is filled with fluid, C. Tooth after chemical technique, the fractured file is smaller in size and can be easily bypassed.
2.4. Vickers microhardness test
The external surfaces of the palatal roots were longitudinally grooved using a diamond disc in a straight micromotor handpiece at a speed of 1500 rpm with a steady flow of water coolant [15]. With a chisel and hammer, each root specimen was broken into two segments; the segment with the fewest surface aberrations on dentin was selected for the test and horizontally inserted into an auto-polymerising acrylic disc.
The surface hardness of the root dentin was determined using a digital Vickers hardness tester with a 100 g load for 15 s [16]. The indentation was performed using a diamond indenter, and the indented area was scanned using a microscopic lens (400 × ) connected to a Vickers tester. Microhardness measurements were performed for each sectioned root at three separate locations (coronal, middle, and apical thirds; 3, 6, and 9 mm from the root tip) [17,18], and an indentation was made on the dentin surface. The representative hardness value at each point was obtained as the average of three indentations to produce a single value. All readings were obtained by the same examiner using the same calibration machine.
2.5. Energy dispersive spectroscopy
Sputter-coated samples were prepared, and imaging and microanalysis were performed on a Bruker Nano (X flash detector 410- M, Germany) at 3 keV; three measurements were performed for each root canal at 3, 6, and 9 mm from the apex (nine measurements for each root canal) [9].
2.6. Statistical analysis
The data were analysed using SPSS (version 25, IBM Corporation, USA), and the statistical analysis distribution was checked using the D'Agostino–Pearson normality test. One-way analysis of variance (ANOVA) and Tukey tests (P < 0.05) were employed to compare the hardness of the teeth before and after both the electrochemical and chemical dissolution tests because they had a normal distribution (Table 1).
Table 1.
Descriptive statistics of the Vickers hardness number (VHN) and analysis of variance (ANOVA) test for the experimental groups.
| Descriptive statistic |
ANOVA |
|||||
|---|---|---|---|---|---|---|
| Group | Level | N | Mean VHN | Std. Deviation | P-value | Significance |
| Control | Coronal | 10 | 65.33 | 0.69929 | <0.001 | HS |
| Middle | 10 | 62.28 | 0.56725 | |||
| Apical | 10 | 55.33 | 0.80561 | |||
| Electrochemical | Coronal | 10 | 65.25 | 0.8182 | <0.001 | HS |
| Middle | 10 | 62.14 | 0.63105 | |||
| Apical | 10 | 55.01 | 0.3414 | |||
| Chemical | Coronal | 10 | 65.19 | 0.86852 | <0.001 | HS |
| Middle | 10 | 62.09 | 0.48865 | |||
| Apical | 10 | 55 | 0.8165 | |||
P < 0.001; HS, highly significant.
Interfacial analytical tests were performed for the coronal, middle, and apical thirds of the teeth using each technique to determine any statistical differences before and after the dissolution techniques (Table 1, Table 2). In addition, interfacial analytical tests were performed between the coronal, middle, and apical thirds of the teeth using each technique to determine the statistical differences between the tested techniques in each subgroup (Table 3).
Table 2.
Tukey's test for the tested techniques.
| Tukey HSD | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable | Mean Difference (I-J) | Std. Error | P-value | Sig | ||
| Control | Coronal | Electrochemical | −7.18000* | 0.22179 | 0.000 | HS |
| Chemical | −10.09000* | 0.22179 | 0.000 | HS | ||
| E | Chemical | −2.91000* | 0.22179 | 0.000 | HS | |
| Electrochemical | Coronal | Electrochemical | −7.10000* | 0.17097 | 0.000 | HS |
| Chemical | −10.13000* | 0.17097 | 0.000 | HS | ||
| E | Chemical | −3.03000* | 0.17097 | 0.000 | HS | |
| Chemical | Coronal | Electrochemical | −7.16000* | 0.26612 | 0.000 | HS |
| Chemical | −10.09000* | 0.26612 | 0.000 | HS | ||
| E | Chemical | −2.93000* | 0.26612 | 0.000 | HS | |
P < 0.001, HS, highly significant.
Table 3.
Analysis of variance (ANOVA) test for the coronal, middle, and apical third of the teeth for the tested techniques.
| ANOVA |
Sig | ||||||
|---|---|---|---|---|---|---|---|
| Sum of Squares | Df | Mean Square | F | P-value | |||
| Coronal | Between Groups | 0.002 | 2 | 0.001 | 0.006 | 0.994 | NS |
| Within Groups | 4.861 | 27 | 0.180 | ||||
| Total | 4.863 | 29 | |||||
| Middle | Between Groups | 0.041 | 2 | 0.020 | 0.099 | 0.906 | NS |
| Within Groups | 5.533 | 27 | 0.205 | ||||
| Total | 5.574 | 29 | |||||
| Apical | Between Groups | 0.013 | 2 | 0.006 | 0.018 | 0.983 | NS |
| Within Groups | 9.754 | 27 | 0.361 | ||||
| Total | 9.767 | 29 | |||||
P < 0.001.
Analysis of variance (ANOVA) was used to analyse the correlation of means between groups, and Tukey's test for multiple parametric comparisons was performed.
3. Results
Table 1 shows the microhardness values for teeth obtained after the dissolution of the fractured instrument by electrochemical and chemical dissolution techniques with the control group. The hardness of the tooth did not change significantly (P > 0.05) because of the dissolving procedures.
Table 1, Table 2 show the results of ANOVA and Tukey's test, respectively, for the tested (9, 6, and 3 mm) coronal, middle, and apical thirds of the teeth in each technique, which demonstrated highly significant differences (P < 0.05) between the groups in the three areas.
Table 4 represents the amount of Ca and P ions in the teeth of the three groups and shows the amount of Ca/P ions in the teeth before and after performing the chemical and electrochemical dissolution techniques.
Table 4.
Descriptive statistics of the Ca & P concentrations and Ca/P ratio in the teeth before and after chemical and electrochemical dissolutions.
| Descriptive Statistics |
Ca/p | Mean | Standard Deviation | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | |||||
| Ca | Control | 10 | 4.584 | 0.00516 | Control | 1.0854 | 0.00182 |
| E | 10 | 4.581 | 0.00738 | ||||
| CH | 10 | 4.579 | 0.00738 | E | 1.08 | 0.00219 | |
| p | Control | 10 | 4.225 | 0.00527 | |||
| E | 10 | 4.223 | 0.00483 | CH | 1.0843 | 0.00168 | |
| CH | 10 | 4.223 | 0.00483 | ||||
Ca, Calcium; P, Phosphate; E, Electrochemical; CH, Chemical.
4. Discussion
The null hypothesis was rejected as no alteration was observed in the Vickers hardness number between the control and experimental groups following both the electrochemical and chemical dissolution techniques, with no change in the chemical composition of dentin. Determination of the microhardness of teeth provides indirect evidence of a change in the mineral content of dental hard tissues [19]. Thus, microhardness determination is the initial step in evaluating dentin behaviour under stress [19]. This study was designed to verify the effects of chemical and electrochemical dissolution techniques on tooth microhardness in the management of fractured NiTi files in the palatal roots of human maxillary molars.
The results confirmed the main premise, as shown in Table 1, Table 3, which indicated that the dissolution procedure did not cause a significant loss in dentin hardness, with no significant differences from the control group. The novel solution [NaF + NaCl2 + MgCl2 + CaCl2] with a pH of 5.0 used in this study, acted by partially dissolving the NiTi instrument components due to its acidity and ions contained in the solution, which acted mainly on Ni ions to restore the original canal. Furthermore, the solution demonstrated low cytotoxicity when tested on human fibroblasts in another study [9] and required less time to bypass the fractured file, demonstrating the ability to be used in a simple chemical dissolution technique. Moreover, the solution can be used with electrochemical techniques for a short clinical duration demonstrating promising results, as confirmed in a pilot study.
CaCl2 and MgCl2 were added to promote Ni dissolution because Ca+ and Mg + have a strong reactivity with Ni in the presence of hydrogen [20], which increases the dissolution process. In addition, the actions of Na+, F+, and Cl− on Ti at pH 5 play an important role. Although the chemical solution used for dissolution contained NaF, with the possibility of fluoride absorption by the root, this did not occur. This may be attributed to the presence of the smear layer, which could have inhibited the absorption of the fluoride (12,000 ppm) that was in contact with the dentin walls for 30 min according to a previous study [21]. Mineralised collagen matrix residues, endodontic sealants, and root canal irrigants are commonly observed in the smear layer [22]. In addition, the smear layer of the root canal walls may be protected by forming a barrier [23]. Buchalla, Lennon, Becker, Lucke, and Attin (2007) investigated whether the smear layer had an impact on the dentinal absorption of fluoride at 12,000 ppm with pH 4.0 [24]. According to their findings, the presence or absence of a smear layer interferes with fluoride absorption in bovine dentinal specimens. The buffering action of dentin should also be highlighted when it comes to pH. Macedo et al. investigated the differences when dentin was exposed to sodium hypochlorite concentrations at various pH values for 1 h, to a pH 5.0 NaOCl solution which was discovered to potentially act as a buffer [25].
′When NiTi files are inserted into electrolytes, the instrument acts as an electron conductor and the electrolyte (novel solution) as a medium for electrical current transport; thus, the electrode reaction describes the phase boundary reaction equivalent to the charge exchange between the instrument and electrolyte [26]. For the electrochemical technique, the file attached by the WE and immersed in the novel solution in the presence of another electrode gold wire with diameter 0.5 mm was connected to CE + RE immersed in the same solution. Both the electrodes were attached to specially designated device to control voltage to 4 V for 10 min and can register the anodic current reaction during the test; thus, in the electrolyte solution, positively charged ions of metal movement will be towards the anodic partial reaction leaving the electrons with a negative charge in the metal (i.e., the electron conductor), indicating oxidization of the metal atoms. In the cathodic partial reaction, the dissolved metal ions at the phase boundary gather electrons and deposit themselves as metal atoms on the electrode surface [27].
In this study, a significant regional difference was observed between the coronal and apical thirds of the teeth (Table 2, Table 3). The microhardness decreased apically, which may be explained by the histology of the relative characteristics of the apical dentin and the patterns of the root canal dentin. Ballal et al. (2010) [28] found that microhardness increased from the apical to the coronal thirds. According to Inoue et al. (2013) [29], mechanical properties are proportional to the tissue mineral content, and coronal dentin contains more minerals than radicular dentin; coronal dentin hardness was approximately 0.8 GPa, which is higher than radicular dentin hardness.
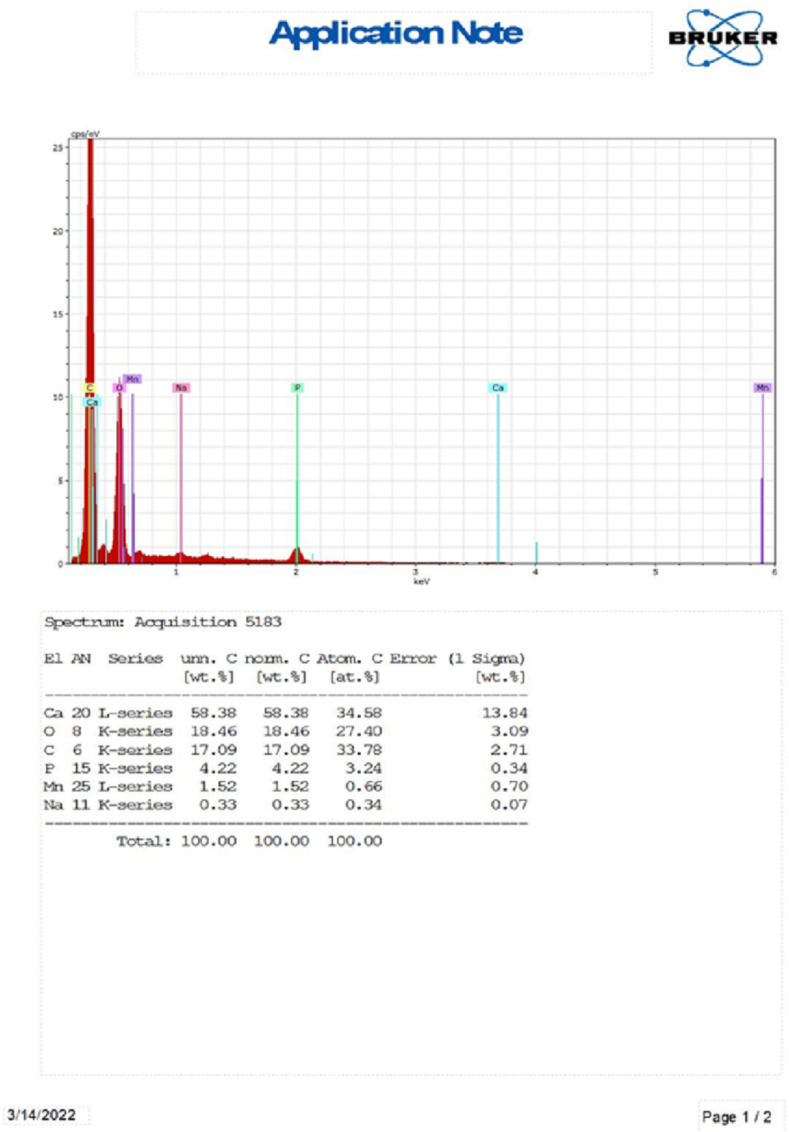
The EDX test of the teeth revealed the presence of different chemical elements (Fig. 3), and multiple comparison tests were performed between the groups, which did not show statistically significant differences. The Ca/P ratio remained practically constant (Table 4). Further studies are warranted to confirm the effect of this novel solution and dissolution technique on the tooth microhardness and Ca/P ratio in dentin to confirm the reliability of the results.
Fig. 3.
Energy-dispersive X-ray of the dentinal tubules of the teeth after electrochemical dissolution.
5. Conclusion
No effect on the dentinal microhardness and alteration in the Ca/P concentration were observed following treatment of the fractured NiTi file in the extracted teeth using the novel chemical solution with both the chemical and electrochemical techniques.
6. Funding and conflict of interest
This research project was self-funded.
7. Data availability statement
The data supporting the findings of this study have not been deposited in a publicly available repository. The data are available upon request.
CRediT authorship contribution statement
Linz Ali Shalan conceived the presented idea, developed the theory, performed computations, and verified the analytical methods and writing. Hussain F. Al-Huwaizi supervised the methodology and findings of this study and wrote, reviewed and edited the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the department of Restorative and Aesthetic Dentistry, College of Dentistry, University of Baghdad, Iraq.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35902.
Contributor Information
Linz Ali Shalan, Email: linz.ali@codental.uobaghdad.edu.iq, linza.shalan@gmail.com.
Hussain F. Al-Huwaizi, Email: Hussain_adp@yahoo.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Souter Nigel J., Messer Harold H. Complications associated with fractured file removal using an ultrasonic technique. J. Endod. 2005;31(6):450–452. doi: 10.1097/01.don.0000148148.98255.15. [DOI] [PubMed] [Google Scholar]
- 2.Terauchi Y., Oleary L., Suda H. Removal of separated files from root canals with a new file removal system: case reports. J. Endod. 2006;32:789–797. doi: 10.1016/j.joen.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Madarati A.A., Hunter M.J., Dummer P.M.H. Management of intracanal separated instruments. J. Endod. 2013;39:569–581. doi: 10.1016/j.joen.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 4.Amaral C.C., Ormiga F., Gomes J.A. Electrochemical-induced dissolution of stainless steel files. Int. Endod. J. 2015;48:137–144. doi: 10.1111/iej.12292. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q., Shen Y., Huang D., Zhou X., Gao Y., Haapasalo M. Evaluation of two trephine techniques for removal of fractured rotary nickel-titanium instruments from root canals. J. Endod. 2017;43:116–120. doi: 10.1016/j.joen.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Ormiga F., da Cunha Ponciano Gomes J.A., de Araujo M.C.P. Dissolution of nickel-titanium endodontic files via an electrochemical process: a new concept for future retrieval of fractured files in root canals. J. Endod. 2010;36:717–720. doi: 10.1016/j.joen.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Aboud L.R., Ormiga F., Gomes J.A. Electrochemical induced dissolution of fragments of nickel-titanium endodontic files and their removal from simulated root canals. Int. Endod. J. 2014;47:155–162. doi: 10.1111/iej.12126. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso L.R., Baldasso F.E.R., Delai D., Montagner F., Kopper P.M.P. Effect of EDTA, sodium, and calcium hypochlorite on the inorganic component of root canal dentin: a SEM analysis. Microsc. Res. Tech. 2019;82:128–133. doi: 10.1002/jemt.23151. [DOI] [PubMed] [Google Scholar]
- 9.Amaral C.C.F., Ormiga F., Boldrini L.C., Miranda P.G., Mendonça T.A., Granjeiro J.M., Gomes J.A.C.P. Evaluation of the effects of the solution used for electrochemical dissolution of nickel-titanium endodontic files on dentine structure, microhardness and cell viability. Int. Endod. J. 2018;51:1434–1445. doi: 10.1111/iej.12950. [DOI] [PubMed] [Google Scholar]
- 10.Amaral C., Ormiga F., Araújo O.M.O., Lopes R.T., Gomes J.A.C.P. Electrochemical dissolution of nickel‐titanium instrument fragments in root canals of extracted human maxillary molars using a small reservoir of electrolyte. Int. Endod. J. 2020;53:1559–1568. doi: 10.1111/iej.13381. [DOI] [PubMed] [Google Scholar]
- 11.Aldury L.A., Ali A.H., Mannocci F. Reciprocation and rotation nickel-titanium file systems' cyclic fatigue and centering ability in premolars accessed by ultraconservative and traditional access cavities: an in vitro study. Saudi Endodontic Journal. 2024;14:44–50. doi: 10.4103/sej.sej_80_23. [DOI] [Google Scholar]
- 12.Abdulrazaq L.A., Ali A.H., Foschi F. Minimally invasive access cavities in endodontics. Journal of Baghdad College of Dentistry. 2023;2:65–75. doi: 10.26477/jbcd.v35i2.3406. [DOI] [Google Scholar]
- 13.Kadhem D.J., Al Haidar A.H.M.J. Antibacterial and cytotoxic effect of a novel biological Nano-silver fluoride synthesized from moringa oleifera leaf extract. Journal of Baghdad College of Dentistry. 2023;35:32–44. doi: 10.26477/jbcd.v35i2.3397. [DOI] [Google Scholar]
- 14.Kowalczuck A., Silva Neto U.X., Fariniuk L.F., Westphalen V.P.D., Laurindo C.A.H., Carneiro E. Electrochemical dissolution of fractured nickel-titanium instruments in human extracted teeth. Int. Endod. J. 2017;50:578–585. doi: 10.1111/iej.12654. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-filho A.M., Sousa-Neto M.D., Savioli R.N., Silva R.G., Vansan L.P., Pecora J.D. Effect of chelating solutions on the microhardness of root canal lumen dentin. J. Endod. 2011;37:358–362. doi: 10.1016/j.joen.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Du W., Zhou X., Yu H. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014;6:61–69. doi: 10.1038/ijos.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saghiri M.A., Delvarani A., Mehrvarzfar P., Malganji G., Lotfi M., Dadresanfar B., Saghiei A.M., Dadvand S. A study of the relation between erosion and microhardness of root canal dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;108:29–34. doi: 10.1016/j.tripleo.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 18.Baldasso S., Roleto L., Da-silva V., Morgental R., Kopper P. Effect of final irrigation protocols on microhardness reduction and erosion of root canal dentin. Braz. Oral Res. 2017;31:e40. doi: 10.1590/1807-3107BOR-2017.vol31.0040. [DOI] [PubMed] [Google Scholar]
- 19.Chabuk M.M., Al-Shamma A.M. Surface roughness and microhardness of enamel white spot lesions treated with different treatment methods. Heliyon. 2023;9(7) doi: 10.1016/j.heliyon.2023.e18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly J.J., Wiswall R.H.J.I.C. Reaction of hydrogen with alloys of magnesium and copper. Inorg. Chem. 1967;6:2220–2223. doi: 10.1021/ic50058a020. [DOI] [Google Scholar]
- 21.Kowalczuck A., Borges M.M., Kruger H., Piasecki L., da Silva Neto U.X., Westphalen V.P.D., Laurindo C.A.H., Carneiro E. Microscopic evaluation of the dentinal walls of extracted human teeth following electrochemical dissolution of fragmented nickel-titanium instruments. Microsc. Res. Tech. 2019;82:1529–1534. doi: 10.1002/jemt.23317. [DOI] [PubMed] [Google Scholar]
- 22.Pashley D.H., Michelich V., Kehl T. Dentin permeability: effects of smear layer removal. J. Prosthet. Dent. 1981;46:531–537. doi: 10.1016/0022-3913(81)90243-2. [DOI] [PubMed] [Google Scholar]
- 23.Violich D.R., Chandler N.P. The smear layer in endodontics – a review. Int. Endod. J. 1998;43:2–15. doi: 10.1111/j.1365-2591.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 24.Buchalla W., Lennon A.M., Becker K., Lucke T., Attin T. Smear layer and surface state affect dentin fluoride uptake. Arch. Oral Biol. 2007;52:932–937. doi: 10.1016/j.archoralbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Macedo R.G., Herrero N.P., Wesselink P., Versluis M., Van Der Sluis L. Influence of the dentinal wall on the pH of sodium hypochlorite during root canal irrigation. J. Endod. 2014;420:1005–1008. doi: 10.1016/j.joen.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Hoque Md Enamul, Showva Nazmir-Nur, Ahmed Mansura, Rashid aAdib Bin, Sadique Sarder Elius, El-Bialy Tarek, Xue Huaizhong. Titanium and titanium alloys in dentistry: current trends, recent developments, and future prospects. Heliyon. 2022;8(11) doi: 10.1016/j.heliyon.2022.e11300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Yahia L., Manceur A., Chaffraix P. Bioperformance of shape memory alloy single crystals. Bio Med. Mater. Eng. 2006;16(2):101–108. [PubMed] [Google Scholar]
- 28.Ballal N.V., Mala K., Bhat K.S. Evaluation of the effect of maleic acid and ethylenediaminetetraacetic acid on the microhardness and surface roughness of human root canal dentin. Endod Journal. 2010;36:1385–1388. doi: 10.1016/j.joen.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Inoue K., Saito M., Yamomoto M., Nishimura F., Miuazaki T. Mineral density of coronal and radicular dentin. Dent. Res. 2013;33:248–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study have not been deposited in a publicly available repository. The data are available upon request.