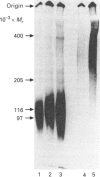
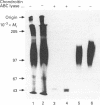
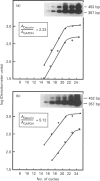
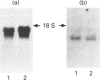
Abstract
Interleukin-4 (IL-4) is a pleiotropic cytokine expressed by inflammatory cells. Previous work from our laboratory has shown that it stimulates collagen synthesis in fibroblasts. Here we report the effects of recombinant human IL-4 on glycosaminoglycan (GAG) and proteoglycan synthesis in normal dermal fibroblasts from adult donors. IL-4 (10 and 100 units/ml) induced a dose-dependent increase of [3H]glucosamine and [35S]sulphate incorporation into total GAGs. The analysis of the different GAG fractions indicated the enhanced synthesis of dermatan/chondroitin sulphates. IL-4 had no effect on hyaluronan synthesis. The increase of sulphated GAG synthesis was correlated with an increase of proteoglycans in the culture medium. Decorin was identified as the major chondroitin/dermatan sulphate-containing proteoglycan in the culture medium of fibroblasts. Its synthesis was strongly stimulated by IL-4. Both the core-protein synthesis and mRNA expression were enhanced, indicating that the cytokine acted, at least in part, at the pre-translational level. These results indicate that IL-4 is able to modulate not only collagen, but also proteoglycan, production by human fibroblasts. Their implications in physiopathological processes such as wound healing or fibrosis is suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartold P. M., Wiebkin O. W., Thonard J. C. Molecular weight estimation of sulfated glycosaminoglycans in human gingivae. Connect Tissue Res. 1982;9(3):165–172. doi: 10.3109/03008208209160257. [DOI] [PubMed] [Google Scholar]
- Bassols A., Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988 Feb 25;263(6):3039–3045. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Breuer B., Schmidt G., Kresse H. Non-uniform influence of transforming growth factor-beta on the biosynthesis of different forms of small chondroitin sulphate/dermatan sulphate proteoglycan. Biochem J. 1990 Jul 15;269(2):551–554. doi: 10.1042/bj2690551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dessen P., Fondrat C., Valencien C., Mugnier C. BISANCE: a French service for access to biomolecular sequence databases. Comput Appl Biosci. 1990 Oct;6(4):355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- Fertin C., Nicolas J. F., Gillery P., Kalis B., Banchereau J., Maquart F. X. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol. 1991;37(8):823–829. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Fleischmajer R., Fisher L. W., MacDonald E. D., Jacobs L., Jr, Perlish J. S., Termine J. D. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol. 1991 Feb;106(1):82–90. doi: 10.1016/1047-8477(91)90065-5. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillery P., Fertin C., Nicolas J. F., Chastang F., Kalis B., Banchereau J., Maquart F. X. Interleukin-4 stimulates collagen gene expression in human fibroblast monolayer cultures. Potential role in fibrosis. FEBS Lett. 1992 May 18;302(3):231–234. doi: 10.1016/0014-5793(92)80448-p. [DOI] [PubMed] [Google Scholar]
- Gillery P., Serpier H., Polette M., Bellon G., Clavel C., Wegrowski Y., Birembaut P., Kalis B., Cariou R., Maquart F. X. Gamma-interferon inhibits extracellular matrix synthesis and remodeling in collagen lattice cultures of normal and scleroderma skin fibroblasts. Eur J Cell Biol. 1992 Apr;57(2):244–253. [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Gold M. R., Duronio V., Saxena S. P., Schrader J. W., Aebersold R. Multiple cytokines activate phosphatidylinositol 3-kinase in hemopoietic cells. Association of the enzyme with various tyrosine-phosphorylated proteins. J Biol Chem. 1994 Feb 18;269(7):5403–5412. [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Kotanides H., Reich N. C. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science. 1993 Nov 19;262(5137):1265–1267. doi: 10.1126/science.7694370. [DOI] [PubMed] [Google Scholar]
- Kresse H., Hausser H., Schönherr E. Small proteoglycans. Experientia. 1993 May 15;49(5):403–416. doi: 10.1007/BF01923585. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Castle B. E., Christiansen J., Schreurs J., Rennick D., Arai N., Hoy P., Takebe Y., Howard M. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol. 1988 Jan 15;140(2):456–464. [PubMed] [Google Scholar]
- Monroe J. G., Haldar S., Prystowsky M. B., Lammie P. Lymphokine regulation of inflammatory processes: interleukin-4 stimulates fibroblast proliferation. Clin Immunol Immunopathol. 1988 Nov;49(2):292–298. doi: 10.1016/0090-1229(88)90119-5. [DOI] [PubMed] [Google Scholar]
- Nietfeld J. J. Cytokines and proteoglycans. Experientia. 1993 May 15;49(5):456–469. doi: 10.1007/BF01923589. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Holness M. A., Katai H., Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest. 1992 Oct;90(4):1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Seyer J. M. Fibroblast chemotaxis induction by human recombinant interleukin-4. Identification by synthetic peptide analysis of two chemotactic domains residing in amino acid sequences 70-88 and 89-122. J Clin Invest. 1991 Jun;87(6):2147–2152. doi: 10.1172/JCI115247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U., Glössl J., Kresse H. Comparison of small proteoglycans from skin fibroblasts and vascular smooth-muscle cells. Biochem J. 1986 Sep 1;238(2):465–474. doi: 10.1042/bj2380465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M. L., Katz D. H. Regulation of the murine Fc epsilon RII (CD23) gene. Functional characterization of an IL-4 enhancer element. J Immunol. 1994 Apr 1;152(7):3453–3466. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Santra M., Danielson K. G., Iozzo R. V. Structural and functional characterization of the human decorin gene promoter. A homopurine-homopyrimidine S1 nuclease-sensitive region is involved in transcriptional control. J Biol Chem. 1994 Jan 7;269(1):579–587. [PubMed] [Google Scholar]
- Schindler C., Kashleva H., Pernis A., Pine R., Rothman P. STF-IL-4: a novel IL-4-induced signal transducing factor. EMBO J. 1994 Mar 15;13(6):1350–1356. doi: 10.1002/j.1460-2075.1994.tb06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992 Jun;6(9):2639–2645. [PubMed] [Google Scholar]
- Sempowski G. D., Beckmann M. P., Derdak S., Phipps R. P. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994 Apr 1;152(7):3606–3614. [PubMed] [Google Scholar]
- Tokunaga K., Nakamura Y., Sakata K., Fujimori K., Ohkubo M., Sawada K., Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987 Nov 1;47(21):5616–5619. [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrowski Y. Effect of hyperthermia on the extracellular matrix. I. Heat enhances hyaluronan and inhibits sulphated glycosaminoglycan synthesis. FEBS Lett. 1993 Nov 8;334(1):121–124. doi: 10.1016/0014-5793(93)81695-v. [DOI] [PubMed] [Google Scholar]
- Wessler E. Electrophoresis of acidic glycosaminoglycans in hydrochloric acid: a micro method for sulfate determination. Anal Biochem. 1971 May;41(1):67–69. doi: 10.1016/0003-2697(71)90192-8. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Antonsson P., Malmström A., Heinegård D., Oldberg A. The synthesis of a family of structurally related proteoglycans in fibroblasts is differently regulated by TFG-beta. Matrix. 1991 Jun;11(3):177–183. doi: 10.1016/s0934-8832(11)80156-3. [DOI] [PubMed] [Google Scholar]
- Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn. 1993 Jun;43(6):283–293. doi: 10.1111/j.1440-1827.1993.tb02569.x. [DOI] [PubMed] [Google Scholar]