Next-generation sequencing (NGS) technology has dramatically enhanced genomic characterization of hematologic malignancies, aiding in diagnosis, risk stratification, and treatment.1-3 Simultaneously, enhanced sequencing and more accessible germline testing has revealed that cancer predisposition syndrome (CPS) accounts for a greater proportion of pediatric cancer diagnoses than previously appreciated.4,5 The diagnosis of a CPS in children is critical for informed treatment decision-making, future cancer surveillance, testing for family members, and family planning.6-8 Although somatic DNA-based panel results may suggest the presence of a germline variant associated with cancer predisposition based on variant allele fraction (VAF) and/or other features of the variant and gene,9 tumor-only sequencing cannot definitively distinguish somatic and germline alterations. Thus, tumor-only sequencing requires follow-up testing for assessment of potential germline mutations. Alternatively, up-front paired tumor and germline (referred to herein as tumor-normal [T/N]) testing at the time of diagnosis utilizes DNA isolated from both cancer cells and non-malignant cells (usually from skin biopsy), sequenced at the same time and on the same platform, such that data from tumor and normal DNA of the same individual can be analyzed together. This approach not only enhances the precision of identifying somatic alterations by comparing the patient’s cancer genome to their own constitutional genome instead of a generic human reference genome, but can also simultaneously identify germline cancer predisposition within genes being sequenced.10 We herein describe the implementation of DNA-based paired T/N testing as part of a large pediatric cancer program, compare diagnostic yield of tumor-only testing followed by germline confirmation versus paired T/N testing, assess clinical implications for patients diagnosed with a CPS, and examine the benefits and limitations of these two sequencing approaches.
A total of 1,190 pediatric and adolescent and young adult (AYA) patients (age 0-35 years) were retrospectively included in this cohort, 1,034 of whom underwent tumor-only testing between June 2016 and October 2022, and 156 of whom underwent paired T/N testing between January 2021 and August 2023 (Figure 1). This study was approved by the Children’s Hospital of Philadelphia Institutional Review Board. Our targeted hematologic cancer panel (HEMEP)2 and comprehensive hematologic panel (COHEM) interrogate 117 known cancer genes associated with hematologic malignancies for SNV, indel, and CNV. The COHEM panel also includes RNA-based fusion analysis for over 700 exons of 117 cancer genes for known and novel fusions.2,11 The identified variants were categorized according to the guidelines.12,13 Demographics and clinical characteristics of this patient cohort are described in Online Supplementary Table S1. Mean age was 9 years. The most common diagnosis was B-cell acute lymphoblastic leukemia (B-ALL; 58%), followed by acute myeloid leukemia (AML) / myeloid sarcoma (18%), and T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL/T-LL; 9%). Most cases (1,068/1,190; 90%) underwent COHEM panel testing, while a smaller proportion had HEMEP panel sequencing (122/1,190; 10%).
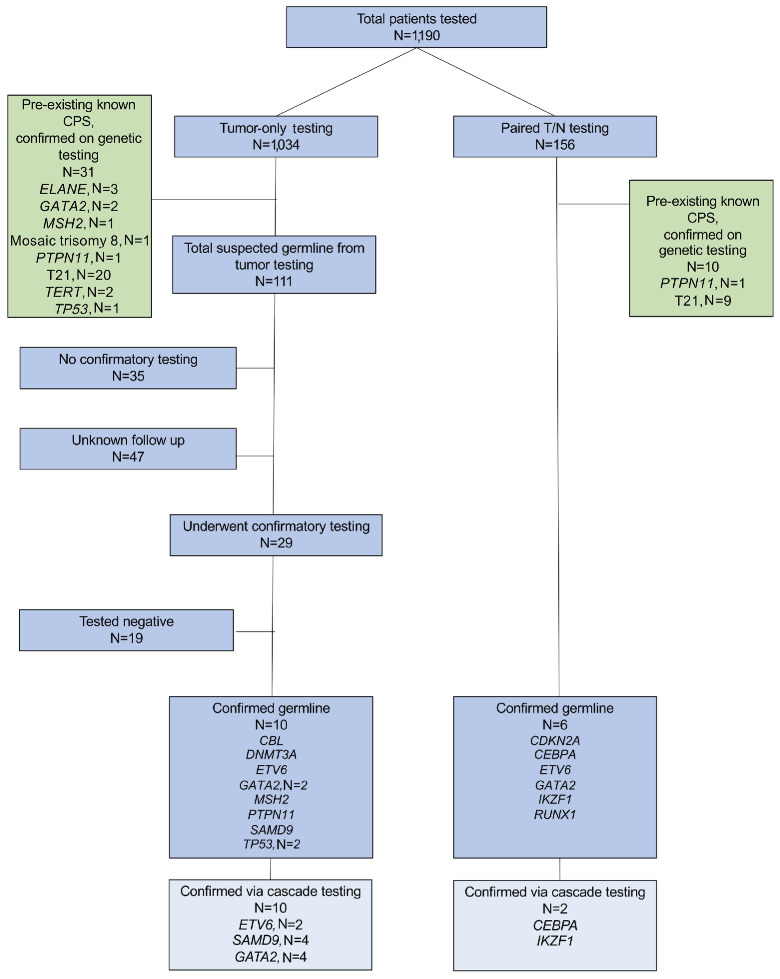
Among 1,034 patients who initially underwent tumor-only molecular sequencing, 31 (3%) patients were found to have genomic alterations consistent with a pre-existing/ known CPS. An additional 111 (11%) patients without a known CPS met criteria for follow-up germline testing as recommended on their diagnostic tumor NGS reports (Figure 1). Within the cohort of 111 patients recommended for germline follow-up testing, 47 cases were submitted from other institutions and had no follow-up clinical data available. Notably, none of these cases had germline specimens submitted to the CHOP diagnostic genomics laboratory. In the cohort of patients treated at our institution (N=64) recommended for follow-up testing, 29 (45%) had subsequent confirmatory germline testing facilitated by the cancer predisposition team or the patient’s primary oncologist, whereas 35 patients (55%) had no documented confirmatory testing. Of these 35 patients, 16 died within six months of somatic testing, which may have precluded the possibility or intention to perform recommended follow-up testing. Notably, this high mortality rate may in part reflect the over-representation of children and AYA at our institution with relapsed / refractory disease, referred for early-phase clinical trial participation or other salvage therapies, who undergo molecular testing. One patient has germline testing pending insurance authorization, and another patient was recently referred to the cancer predisposition clinic. For the remaining 17 patients, germline predisposition was mentioned only in genomics reports, suggesting the information may have potentially been missed by clinicians and families. Among the 29 patients who initially underwent somatic-only tumor testing and subsequent recommended germline testing, 10 (34%) were confirmed to have a CPS, including genetic mutations associated with Noonan syndrome-like disorder (CBL), DNMT3A overgrowth syndrome and predisposition to hematologic malignancy/ Tatton-Brown-Rahman syndrome (DNMT3A), ETV6 thrombocytopenia and leukemia predisposition syndrome (ETV6), GATA2 deficiency syndrome (GATA2), Lynch syndrome (MSH2), Noonan syndrome (PTPN11), Mirage syndrome (SAMD9), and LFS (TP53).
Figure 1.
Consort diagram of patients undergoing genomic testing with tumor-only or paired tumor-normal testing and cancer predisposition syndrome diagnoses.
Among 156 patients who underwent paired T/N testing, 10 patients (6%) were found to have genomic alterations consistent with a pre-existing / known CPS. Six patients (4%) were diagnosed with a new CPS (Figure 1), including CDNK2A-associated predisposition (CDKN2A), CEBPA-associated predisposition to AML (CEBPA), ETV6 thrombocytopenia and leukemia predisposition syndrome (ETV6), GATA2 deficiency syndrome (GATA2), IKZF1-associated leukemia predisposition (IKZF1), and RUNX1 familial platelet disorder with associated myeloid malignancies (RUNX1). If tumor-only testing, instead of T/N, were performed on those 6 patients with a new CPS, the results would trigger a germline testing.
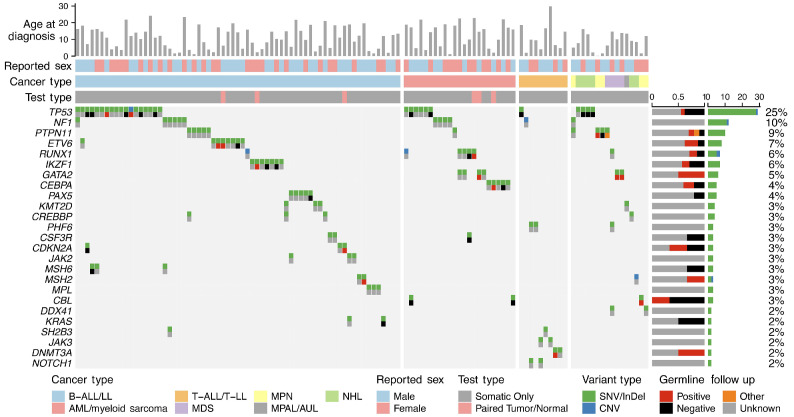
Figure 2.
Oncoprint of variants recommended for germline follow up, identified through somatic testing, or confirmed germline variants, identified through paired tumor/normal testing. Top icon demonstrates variant type (SNV / indel, CNV). Bottom icon demonstrates germline follow-up result. Annotations for age at diagnosis, sex, cancer type, and test type (somatic vs. tumor/normal [T/N]) are displayed on the top of the oncoprint. Barcharts to the right show the proportion of patients with a germline variant identified that underwent follow up by gene (left) and the frequency of each gene within the whole cohort (right).
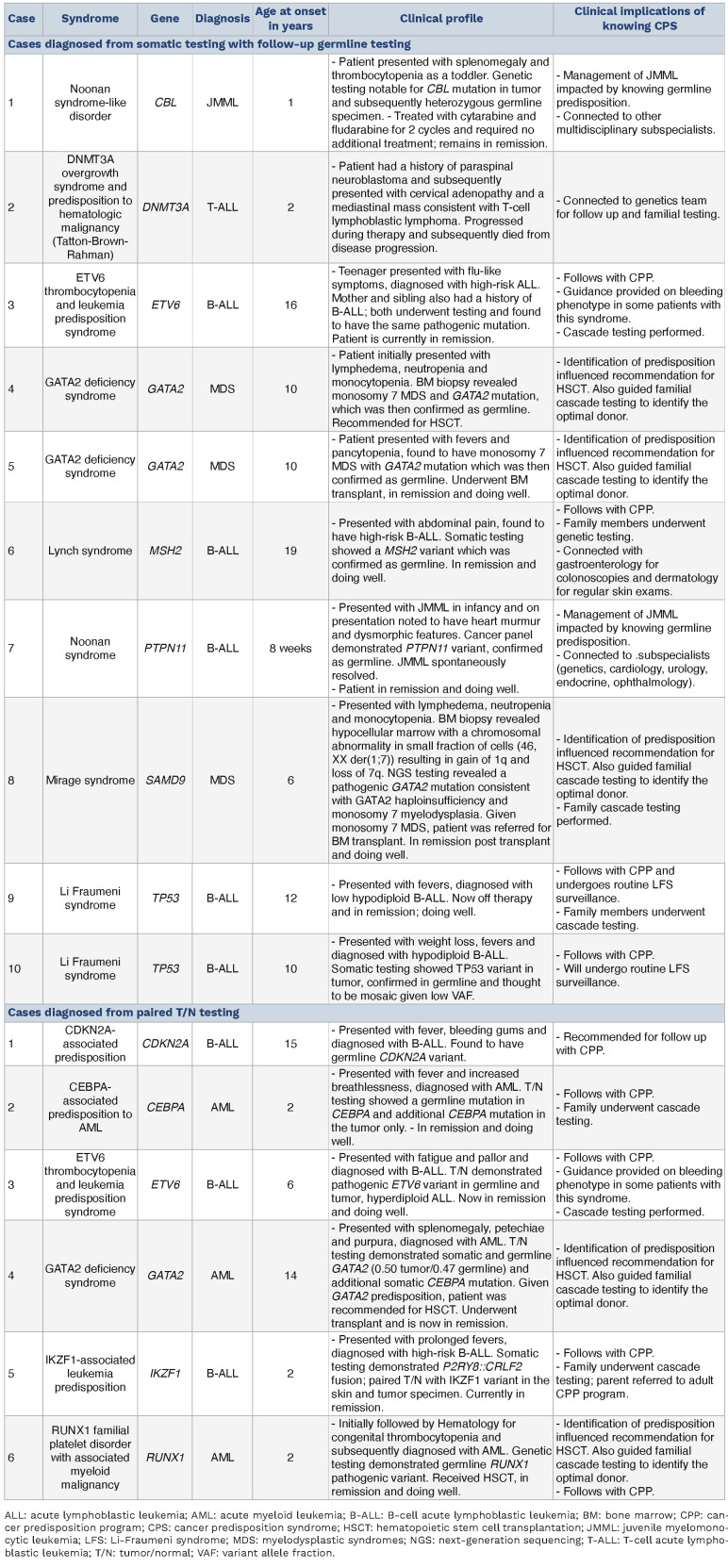
Table 1.
Clinical characteristics of patients diagnosed with cancer predisposition syndrome.
Potential or confirmed germline variants were identified in 35 cancer predisposition genes from 117 patients (Figure 2, Online Supplementary Table S2). Potential germline variants were most frequently identified in TP53 (N=36), NF1 (N=15), and PTPN11 (N=10). Among patients diagnosed with a CPS, there were several immediate, clinically relevant implications (Table 1). Two patients with germline CBL- or PTPN11-mutant juvenile myelomonocytic leukemia (JMML) were treated according to guidelines for patients with germline predisposition to JMML, which differ from guidelines for children with somatic Ras pathway mutation-driven JMML.14 An additional 3 patients were diagnosed with germline GATA2 deficiency syndrome, one with AML and 2 with monosomy 7 myelodysplastic syndromes. All 3 were recommended for hematopoietic stem cell transplantation (HSCT) with genetic testing of family members for optimal GATA2 wild-type transplant donor selection and predisposition screening. Two patients were diagnosed with Li-Fraumeni syndrome (LFS) portending lifelong increased risk of malignancy and prompting referral to the cancer predisposition team for optimal tumor surveillance, genetic counseling, and cascade testing for at-risk family members. Of the 16 patients with hematologic malignancies and a CPS newly diagnosed from genomic testing (Figure 1), there was no known cancer predisposition or early onset cancer in any other immediate family members. To date, 33 of 48 (69%) family members recommended for germline testing based upon diagnoses of a CPS in their probands have undergone testing (Online Supplementary Figure S1). Among those tested, 12 family members tested positive for germline mutations and a CPS (including CEBPA, ETV6, GATA2, IKZF1, SAMD9) with indications for follow up in the pediatric or adult cancer predisposition clinic, and in some instances referral for HSCT.
Overall, 9% of patients in this cohort who underwent tumor-only testing with follow-up germline confirmation or up-front paired T/N testing (10 of 29 and 6 of 156, respectively) at our institution were diagnosed with a new CPS, consistent with reported frequency in pediatric cancer.5 Among patients with suspected germline predisposition from somatic tumor-only testing, a substantial number (32%) did not undergo confirmatory germline testing. The results of our study suggest that paired T/N testing for pediatric and AYA patients with hematologic malignancies has several advantages over somatic tumor-only testing. From a patient care perspective, this approach identifies somatic variants and hereditary predisposition simultaneously,10 obviating barriers to germline testing, mitigating loss to follow-up, and reducing undue anxiety for patients with findings suggestive of germline alteration that are, in fact, somatic events and do not have familial implications. Even with improvement in data reporting and recognition of a possible CPS, challenges with insurance approval for testing and additional clinical visits create substantial barriers to obtaining follow-up germline testing for many patients. Furthermore, with declining sequencing costs, there is now minimal extra cost of sequencing a paired normal sample on a single, streamlined platform. Thus, at our institution, the cost of up-front paired T/N is less than the cumulative cost of tumor-only sequencing followed by confirmatory sequencing with specific primer design and lab implementation. From a genomic perspective, paired T/N sequencing allows for the subtraction of variants in matched normal tissue from tumor tissue to reveal acquired genetic alterations that are truly somatic in origin and aid in variant classification.
Our study suggests that up-front paired T/N testing should be pursued, when possible, with thorough pre-test counseling,15 as the impact on clinical decision-making and long-term management is significant when a CPS is identified. Furthermore, this strategy can potentially alleviate undue emotional burden and mitigate economic barriers associated with follow-up testing in most patients with somatic mutation-driven cancers who do not require further germline testing. Future studies should explore patient and family experiences utilizing mixed methods approaches incorporating qualitative data, as well as assess implementation of broader predisposition genomic panels in the context of pediatric malignancies. At present, our comprehensive hematologic panel does not include all cancer predisposition-related genes. We are currently working to implement a more comprehensive panel, as the continuing reduction in sequencing costs enables expanded genomic profiling without additional expense.
Supplementary Material
Funding Statement
Funding: This investigation was supported by 5K12CA076931-24 to HN from the National Institute of Health. SKT is a Scholar of the Leukemia & Lymphoma Society and holds the Joshua Kahan Endowed Chair in Pediatric Leukemia Research at the Children’s Hospital of Philadelphia.
Data-sharing statement
The datasets generated and analyzed for this study are available from the corresponding author on reasonable request.
References
- 1.Duncavage EJ, Bagg A, Hasserjian RP, et al. Genomic profiling for clinical decision making in myeloid neoplasms and acute leukemia. Blood. 2022;140(21):2228-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surrey LF, MacFarland SP, Chang F, et al. Clinical utility of custom-designed NGS panel testing in pediatric tumors. Genome Med. 2019;11(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong Y, Xu F, Wu J, Schubert J, Li MM. Application of next generation sequencing in laboratory medicine. Ann Lab Med. 2021;41(1):25-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody RJ, Wu YM, Lonigro RJ, et al. Integrative clinical sequencing in the management of refractory or relapsed cancer in youth. JAMA. 2015;314(9):913-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373(24):2336-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kratz CP, Achatz MI, Brugières L, et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res. 2017;23(11):e38-e45. [DOI] [PubMed] [Google Scholar]
- 7.Tabori U, Hansford JR, Achatz MI, et al. Clinical management and tumor surveillance recommendations of inherited mismatch repair deficiency in childhood. Clin Cancer Res. 2017;23(11):e32-e37. [DOI] [PubMed] [Google Scholar]
- 8.Xiao H, Shi J, Luo Y, et al. First report of multiple CEBPA mutations contributing to donor origin of leukemia relapse after allogeneic hematopoietic stem cell transplantation. Blood. 2011;117(19):5257-5260. [DOI] [PubMed] [Google Scholar]
- 9.MacFarland SP, Zelley K, Surrey LF, et al. Pediatric somatic tumor sequencing identifies underlying cancer predisposition. JCO Precis Oncol. 2019;3:PO.19.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandelker D, Ceyhan-Birsoy O. Evolving significance of tumornormal sequencing in cancer care. Trends Cancer. 2020;6(1):31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang F, Lin F, Cao K, et al. Development and clinical validation of a large fusion gene panel for pediatric cancers. J Mol Diagn. 2019;21(5):873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MM, Datto M, Duncavage EJ, et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: a Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19(1):4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards S, Aziz N, Bale S, et al. Standards and Guidelines for the Interpretation of Sequence Variants: a Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wintering A, Dvorak CC, Stieglitz E, Loh ML. Juvenile myelomonocytic leukemia in the molecular era: a clinician’s guide to diagnosis, risk stratification, and treatment. Blood Adv. 2021;5(22):4783-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botkin JR. Ethical issues in pediatric genetic testing and screening. Curr Opin Pediatr. 2016;28(6):700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed for this study are available from the corresponding author on reasonable request.