Abstract
Introduction
Poor communication about serious injury in older adults can lead to treatment that is inconsistent with patient preferences, create conflict and strain healthcare resources. We developed a communication intervention called Best Case/Worst Case-intensive care unit (ICU) that uses daily scenario planning, that is, a narrative description of plausible futures, to support prognostication and facilitate dialogue among patients, their families and the trauma ICU team. This article describes a protocol for a multisite, randomised, stepped-wedge study to test the effectiveness of the intervention on the quality of communication (QOC) in the ICU.
Methods and analysis
We will follow all patients aged 50 and older admitted to the trauma ICU for 3 or more days after a serious injury at eight high-volume level 1 trauma centres. We aim to survey one family or ‘like family’ member per eligible patient 5–7 days following their loved ones’ admission and clinicians providing care in the trauma ICU. Using a stepped-wedge design, we will use permuted block randomisation to assign the timing for each site to begin implementation of the intervention and routine use of the Best Case/Worst Case-ICU tool. We will use a linear mixed-effects model to test the effect of the tool on family-reported QOC (using the QOC scale) as compared with usual care. Secondary outcomes include the effect of the tool on reducing clinician moral distress (using the Measure of Moral Distress for Healthcare Professionals scale) and patients’ length of stay in the ICU.
Ethics and dissemination
Institutional review board (IRB) approval was granted at the University of Wisconsin, and all study sites ceded review to the primary IRB. We plan to report results in peer-reviewed publications and national meetings.
Trial registration number
Keywords: palliative care, medical ethics, trauma management, adult intensive & critical care, clinical trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
In this registry-enabled clinical trial, we will use the American College of Surgeons Trauma Quality Improvement Program (ACS TQIP) national registry to follow all eligible patients at eight high-volume level I trauma centres across the USA.
We designed this study to minimise the potential for missing data and anticipate low rates of missingness because family surveys are collected at the time of enrolment and all trauma centres involved in this study report clinical data to the ACS TQIP for quality assurance and benchmarking.
We have a strong implementation strategy, a fidelity-monitoring plan with weekly audit feedback and the ability to verify adherence to the intervention.
We will use a stepped-wedge design, which allows us to test the intervention in a multilevel space while minimising the risk of contamination bias compared with a patient-level randomised design testing the same intervention.
We will not survey patients nor link family surveys to individual patient outcomes, this study design compromise improves study feasibility and reduces regulatory complexity but will limit our ability to interpret some study findings.
Introduction
Background
Each year, half a million adults 50 years or above suffer injury from a fall or other traumatic event.1 2 Older adults fare far worse than younger patients with similar injuries due to chronic comorbid conditions and reduced physiological reserve. As such, traumatic injury is often a preterminal event, with 20% in-hospital and 40% 1-year mortality.3 Treatment for traumatic injury frequently involves burdensome treatments (like invasive surgical procedures or prolonged life support) that may be inconsistent with patients’ preferences and goals.4,6 This disconnect between patients’ priorities and the treatments received can lead to conflict in the intensive care unit (ICU), specifically interpersonal conflict among clinicians (eg, between nurses and surgeons) and with patients’ loved ones (eg, between surrogate decision-makers and the trauma ICU team) during treatment discussions.7 8 Moreover, overtreatment at the end of life (EOL) prolongs dying and contributes US$44 billion annually in the USA to healthcare costs.9 A communication intervention that facilitates the articulation of patient priorities could reduce unwanted invasive procedures and clarify patients’ long-term goals, benefiting patients, loved ones, clinicians and healthcare systems.10
The Best Case/Worst Case-ICU tool
We developed a communication intervention called Best Case/Worst Case-ICU that uses scenario planning, that is, a narrative description of plausible futures, to support decision-making and facilitate dialogue among patients, their loved ones and the trauma team. Typically, in accordance with standards for informed consent, clinicians present risks as discrete complications for isolated physiologic systems (eg, a 50% chance of kidney failure) or the binary outcome of mortality (eg, a 40% chance of survival).11 Because this language does not describe how a patient might experience treatments or the expected downstream outcomes, such as predictable changes in functional status, prolonged recovery or need for long-term care in a nursing home, patients and families may struggle to anticipate and account for the consequences of serious injury and make treatment decisions accordingly. Scenario planning provides an alternative strategy for managing uncertainty that is in distinct contrast to emphasising isolated risks or discrete treatment effects. Instead, scenario planning generates multiple plausible futures, prompting decision-makers to consider causal relationships and visualise a range of outcomes based on sound analysis of the present.12
We designed the Best Case/Worst Case-ICU tool to help visualise uncertainty, illustrate the interplay between major events and prognosis and describe how patients might experience the various treatments received along the course of care. By using a graphical aid to illustrate ‘what we are hoping for’, ‘what we are worried about’,13 and the evolution of the patient’s story or clinical course over time, including setbacks and improvements, the tool aims to keep everyone (clinicians, patients and loved ones) well informed. The tool facilitates clinician delivery of critical prognostic information over the longitudinal course of care, allowing subsequent treatment decisions, for example, additional operations or prolonged mechanical ventilation, to be made within the context of the patient’s overall health status and goals. Ultimately, this tool alerts patients and families to the life-limiting nature of serious injury and provides valuable insight as they consider whether comfort-focused strategies might better support their care needs.
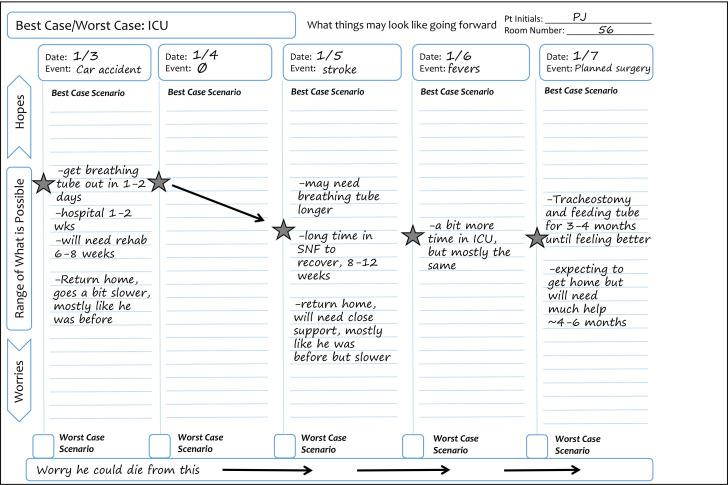
We designed the tool to fit the pace of busy trauma ICU rounds. The trauma team collaboratively completes the graphical aid during the summative systems-based review daily for each patient (figure 1). With usual care, a clinician (typically a surgical resident) lists each physiologic system, that is, neuro, cardiac, pulmonary, etc, or individual medical problems with an assessment and plan for each. When using the tool, they add ‘outlook’, that is, the best-case scenario, at the end. While the attending physician or fellow generates this story, another team member records it on the graphical aid. The worst-case scenario is modified as needed but does not typically require daily updating. The graphical aid is posted in the patient’s room, where loved ones and clinicians can use it to recall what to expect, visualise uncertainty and see how things change over the patient’s course of care.
Figure 1. Example of the Best Case/Worst Case-ICU graphical aid. On each day of a patient’s ICU stay, the trauma team uses a preprinted graphical aid to review major events from the previous 24 hours and describe the patient’s overall health trajectory. On the graphical aid, each ICU day corresponds to a column, and the range of possible scenarios, that is, stories describing how this new injury could play out over time, are designated on a vertical line. A star distinguishes the ‘best-case scenario’ and a box designates the ‘worst-case scenario’. Each day, the trauma team will record any new major events at the top of the column. The star is moved based on how a new event, like a diagnosis of pneumonia or an improvement in neurological function after a stroke, changes the best-case scenario. Over time, the placement of the star goes up or down depending on how these events change the patient’s overall story. Arrows may be used to denote information is carried over from the previous day. SNF, skilled nursing facility.
The daily stories and the graphical aid provide support and perspective for everyone involved in the care of the patient. If the patient clinically improves, their loved ones are primed for the road to recovery. If the patient worsens, their loved ones will be prepared, and the gravity of the patient’s illness will not come as a surprise. Important decisions, such as proceeding with an operation or continuing mechanical ventilation, can be made within the context of the patient’s overall health trajectory. We hypothesise that this will lead to improved communication in the ICU, and patients will receive care that better aligns with their health goals. We theorise this will reduce interpersonal ICU conflict that contributes to clinician burn-out and moral distress.
Methods and analysis
Design and setting
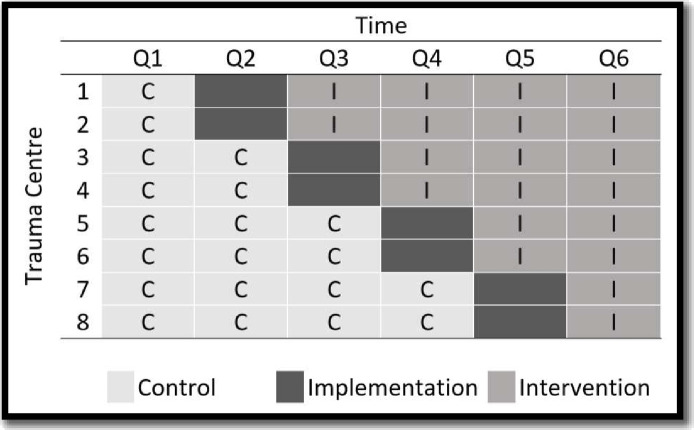
We will use a multisite, randomised, stepped-wedge design to test the effectiveness of the Best Case/Worst Case-ICU tool.14 This 18-month study will be executed over six 3-month long waves (figure 2). In wave 1, all patients will receive the usual care. With each subsequent wave, we will randomly select two sites to enter the implementation phase. Data collection will cease for sites during the implementation wave and the study implementation team will train clinicians to use the Best Case/Worst Case-ICU tool. After the implementation wave, the site will be in the intervention arm, and patients will receive care from a trauma team that routinely uses the Best Case/Worst Case-ICU tool.
Figure 2. Using a stepped-wedge design, we will conduct this study over six 3-month long waves at eight high-volume trauma centres. During the first wave, all patients will receive usual care. With each subsequent wave, two randomly selected sites will enter the implementation phase. Data collection will cease during implementation and the study implementation team will train clinicians to use the Best Case/Worst Case-ICU tool. Following the wave for implementation training, patients will receive care from a trauma team that routinely uses the Best Case/Worst Case-ICU tool. ICU, intensive care unit.
We will conduct this study at eight high-volume level I trauma centres from across the USA (table 1). Data collection began on 1 July 2023 and the estimated date of study completion is 31 December 2025.
Table 1. Study sites with annual number of eligible patients based on 2019 ICU volume.
| Trauma centre | Location | Patients meeting study eligibility criteria | Stratification for randomisation |
| Harborview Medical Center (University of Washington) | Seattle, WA, USA | 702 | Very high |
| University of Alabama at Birmingham | Birmingham, AL, USA | 615 | Very high |
| Grady Memorial Hospital (Morehouse School of Medicine) | Atlanta, GA, USA | 583 | Very high |
| Lehigh Valley Health Network | Allentown, PA, USA | 507 | Very high |
| Rhode Island Hospital | Providence, RI, USA | 504 | High |
| Shock Trauma (University of Maryland Medical Center) | Baltimore, MD, USA | 398 | High |
| Froedtert Hospital (Medical College of Wisconsin) | Milwaukee, WI, USA | 321 | High |
| UC Davis Medical Center | Sacramento, CA, USA | 289 | High |
ALAlabamaCACaliforniaGAGeorgiaICUintensive care unitMDMarylandPAPennsylvaniaRIRhode IslandWAWashingtonWIWisconsin
Participants
Patients
We will follow all patients aged 50 and older admitted to the trauma ICU at study sites for 3 or more days after a serious injury.
Family members
For each patient who receives 3 or more days of ICU care provided primarily by the trauma ICU team, we will invite one family member or informally designated ‘like family’ member or primary surrogate decision-maker (hereafter family) to participate 5–7 days after admission. We will use medical records and nursing referrals to identify the person most frequently engaged in the patient’s care. Family members must be at least 18 years, speak English or Spanish and have decision-making capacity.15 We will approach family members regardless of whether their loved one has been discharged from the ICU or is deceased.
Clinicians
We will invite all clinicians providing care in the trauma ICU to participate in the intervention training. This includes ICU attendings (eg, trauma surgeons), fellows, residents, advance practice providers (APPs), bedside nurses and medical assistants, respiratory and physical therapists, social workers, pharmacists, and chaplains. We will exclude individuals who do not provide primary care in the trauma ICU, for example, medical specialists.
Recruitment
In this registry-enabled study, all patient-level data will come from the American College of Surgeons Trauma Quality Improvement Program (ACS TQIP) national registry, which collects demographics and outcomes for all trauma patients at 850 participating centres according to the National Trauma Data Standards.16 We will not directly recruit patients for this study. A research coordinator (RC) at each site will approach eligible family members in person or via telephone. Qualifying family members will receive a US$20 incentive after a one-time survey completion.
We will send clinicians an anonymous link to an electronic survey via their hospital-based email address, with up to three additional email requests. To increase the response rate, RCs will request survey completion in person in the trauma ICU, during multiple shifts over the 4-week data collection period. Additionally, the site principal investigator (PI) will encourage the completion of study procedures at ICU team meetings and through hospital-generated electronic notification systems (eg, weekly email updates). Clinicians will receive a US$5 incentive for each survey completed (up to US$20 total). Attending surgeons and fellows will receive US$100 for the completion of the 30 min one-on-one training.
Randomisation and blinding
We will use permuted block randomisation to assign the timing for each site to begin implementation of the intervention and routine use of the Best Case/Worst Case-ICU tool. Study sites will be stratified based on historic patient volume (ie, very high or high) to increase the likelihood of a balanced distribution of participants across study arms. A study statistician will link treatment group assignment to patient and family member data using the patient’s admission date.
Family members will be told the goal is to evaluate clinician–patient communication but will be blinded to the specific objectives (ie, that we are testing a graphical aid communication tool) of this study, which may mitigate bias given the nature of our primary outcome.17 18 Clinicians will not be blinded to the treatment group. While we will inform all clinicians of the study goals, clinicians will not be told specific study outcomes or hypotheses. TQIP registrars will abstract data throughout the study, in a manner consistent with their normal work processes, without being informed of the status of interventional procedures. To decrease ascertainment bias, on-site research staff will not participate in intervention implementation and will adhere to a strict study script during interactions with clinicians and family members.
Intervention
Delivery of the intervention requires training trauma ICU teams on how to use the Best Case/Worst Case-ICU tool. The intervention is considered quality improvement because its primary purpose is to integrate guideline-recommended behaviours, for example, timely communication with families/loved ones and emotional support as part of routine care in the ICU.19 20 Because the Best Case/Worst Case-ICU tool is intended for team-based clinician–family communication, the training programme is tailored to the clinician’s role on the trauma team.
We will invite all attending physicians and fellows who round in the trauma ICU to attend a 30 min one-on-one instructional programme, followed by coaching, assessment and additional training, as needed. Instruction for attendings and fellows will focus on translating clinical knowledge and prognostic information into the Best Case/Worst Case-ICU format. Key topics include daily scenario planning to tell a best-case and worst-case scenario, identifying major events that change the best-case scenario and completing the graphical aid while also reviewing skills to support shared decision-making for patients with serious illnesses. Attendings/fellows who do not achieve minimal competence (10 of 14 essential tool elements) on assessment will receive additional training until they reach competence.
For resident trainees and APPs who are rotating in the ICU, we will host a 30 min to 1-hour group session, which includes a 10 min instructional video. This session focuses on teaching how to routinely complete the graphical aid on rounds with minimal disruption, specifically, how to include the patient’s ‘outlook’ and document the best-case scenario. Using a hypothetical case, learners will practice completing the graphical aid and watch a standardised video reviewing the case. We will repeat this training on a regular basis to accommodate new residents brought into the ICU for clinical rotations. For general surgery residents, who often comprise a significant portion of resident trainees in the ICU, we will also offer an institution-wide one-time training.
We will provide education to bedside nurses and other clinical ICU staff during in-service meetings and other routine meetings as guided by on-site nurse managers. Our implementation team will describe the tool, answer questions and reinforce the ‘this is what we are hoping for’ and ‘this is what we are worried about’ dialogue. To accommodate rotating 24/7 schedules, we will display educational posters and brochures directed towards communicating with nurses throughout the ICU and include QR codes with links to instructional videos which detail how to use the tool and provide instructions on supporting family interactions with the graphical aid.
To accommodate staff turnover and attrition, we will provide individual training for attending physicians and fellows who arrive at the institution after the implementation period using virtual one-on-one instruction. We will offer to train an on-site resource nurse champion, to be selected by the on-site nurse manager, for as-needed nurse education.
Following the above intervention training, an implementation liaison (eg, a surgical resident or APP) at each site will continue to monitor and encourage routine use of the tool on rounds twice weekly to observe BC/WC-ICU in use and provide feedback or support for the rounding ICU team during the implementation phase.
Adherence
An implementation liaison, who is separate from the research team that conducts surveys, will perform once-weekly audits comparing the number of study-qualified patients to the number who received daily communication using the Best Case/Worst Case-ICU tool, as assessed by graphical aid completion. The implementation liaison will retain a sample of deidentified graphical aids on digital record and note where each was posted in the ICU. The implementation team will use a scoring rubric to judge the completeness of each graphical aid and provide feedback to clinicians as needed. If we find that routine use of the intervention falls below 80% of eligible patients, we will deploy additional strategies to promote use. Specifically, we will follow up directly with individual trauma surgeons to identify barriers, perform twice-weekly audits and distribute study-wide comparator reports on adherence to each site. With the input of the site PI, we will determine site-specific strategies, such as incorporating prefilled prompts to the graphical aid or providing improvement-based incentives, such as small rewards for high performance, for example, cookies or other treats.
Control
Prior to implementation of the intervention at each site, all patients admitted to the trauma ICU will receive usual care, in accordance with the stepped-wedge study design. The pattern of usual care is well characterised,1121,25 wherein clinician communication often focuses on isolated problems and treatment decisions, which can be disarticulated from the patient’s overall health trajectory, prognosis and long-term functional or cognitive outcomes.
Data collection
TQIP registry
Patient-level data (ie, demographics, clinical data and patient outcomes, including ICU length of stay (LOS)) are collected as part of the ACS TQIP trauma registry.16 To promote quality care, participation in the TQIP registry is required for verification as a level I trauma centre and, independent of their participation in this study, each study site contracts trained registrars to abstract data elements for all patients admitted with a traumatic injury. For this study, ACS TQIP will provide data, without direct patient identifiers, for each study-qualified patient admitted to the hospital during the study period. The ACS developed an incremental data collection platform (IDCP) for RCs to enter one additional variable not currently collected by TQIP (vital status at 6 months) and one data quality check (ICU LOS). After 7–8 months of a patient’s admission to the ICU, RCs will use the patient’s medical record number and trauma ID (provided by TQIP abstractors) to record this information into the ACS IDCP, which will be linked to the TQIP database and the admission of interest. We will not link patient data to family member data collected by the study team, as neither the ACS TQIP provided data nor the family member data will contain Protected Health Information (PHI) (eg, name, date of birth) that would allow us to link the two distinct data sources. The decision to not collect PHI not only safeguards patients’ privacy, it also improves study feasibility as we have found obtaining consent from trauma patients difficult due to their critical condition.
Family member surveys
We will invite one family member per study-eligible patient to complete a one-time questionnaire administered 5–7 days after the patient’s admission. The questionnaire consists of the Quality of Communication (QOC) survey,26 the Receipt of Goal Concordant Care survey27 and demographic questions about the family member and the patient.
Clinician surveys
Three months prior to a site’s implementation wave and again 12 months later, we will ask clinicians to complete the Measure of Moral Distress for Healthcare Professionals (MMD-HP) and Maslach Burnout Inventory (MBI) questionnaires.28 29 To reduce respondent burden, we will administer the two surveys 2 weeks apart, starting with the MMD-HP. We will also collect demographic information from clinicians including race/ethnicity, gender, role in the ICU, time in current position and time employed at the institution. On study completion, we will also ask trauma surgeons to complete the Practitioner Opinion Survey30 to evaluate the use of the intervention clinically.
The Qualtrics data collection platform (V.2023, Qualtrics, Provo, Utah, USA. https://www.qualtrics.com) will be used to store clinician and family survey data and voluntarily provided contact information. All study staff members who have access to identifiable subject information will be HIPAA and Human Subjects trained (eg, Collaborative Institutional Training Initiative (CITI) trained) prior to participating in study recruitment, enrolment, data collection and data analysis.
Outcomes
Primary outcome
We will compare family-reported QOC scores between treatment groups. The QOC instrument includes two subscales, the General QOC and the EOL QOC, wherein items not performed by the clinician receive a score of 0.26 This allows us to discriminate between QOC attributable to satisfaction with the clinician, which often has high ceiling effects, and the QOC about prognosis and outcomes.
Key secondary outcomes
As a proximate measure of the effectiveness of the intervention in reducing ICU conflict, we will compare MMD-HP scores between treatment groups. The MMD-HP multiplies a clinician’s reported frequency of experience and level of distress for situations specifically related to serious illness communication.28 We will also compare treatment groups’ scores on the MBI, which is recommended by the National Academy of Science and Medicine to measure clinician burn-out.31 32
To test the effectiveness of the intervention on patient outcomes, we will compare the mean LOS in the ICU, measured as the cumulative amount of time spent in the ICU post-injury, between treatment groups.
We outline additional secondary outcomes in table 2.
Table 2. Primary and secondary outcomes.
| Construct | Specific measure | Type; range | Source | Timing |
| Primary study outcome | ||||
| Family-reported Quality of Communication (QOC) | The QOC questionnaire, including 6-item general communication subscale and 7-item EOL communication subscale (20 items) | Continuous; 0–10 | Family member survey | 5–7 days after admission |
| Secondary outcomes | ||||
| Family-reported General QOC | The general communication subscale or the QOC questionnaire (6 items) | Continuous; 0–10 | Family member survey | 5–7 days after admission |
| Family-reported End-of-Life (EOL) QOC | The EOL communication subscale or the QOC questionnaire (7 items) | Continuous; 0–10 | Family member survey | 5–7 days after admission |
| Receipt of Goal Concordant Care (GCC) | The GCC survey 2 items: (1) preferences for care and (2) current receipt of care consistent with preferences | Binary; 1/0 | Family member survey | 5–7 days after admission |
| Moral Distress (MMD-HP) | MMD-HP measures the frequency and level of distress of clinician experiences, targeting situations specifically related to serious illness communication. (27 items) | Continuous;0–432 | Clinician survey | T0 and T1* |
| Maslach Burnout Inventory (MBI) | Emotional Exhaustion (EE) subscale of MBI for Medical Personnel (22 items total, 9 items on subscale) | Continuous; EE: 0–54 | Clinician survey | T0 and T1 |
| Depersonalisation (DP) subscale of MBI for Medical Personnel (22 items total, 5 items on subscale) | Continuous; DP: 0–30 | Clinician survey | T0 and T1 | |
| Personal Accomplishment (PA) subscale of MBI for Medical Personnel (22 items total, 8 items on subscale) | Continuous; PA: 0–48 | Clinician survey | T0 and T1 | |
| ICU length of stay | Total time measured in days patient spent receiving ICU care during admission for traumatic injury (not necessarily concurrent) | Continuous (log-transformed) | TQIP chart review | During hospitalisation |
| Total ventilator days | Total time measured in days patient spent on a ventilator during admission for traumatic injury | Continuous | TQIP chart review | During hospitalisation |
| Death | In-hospital patient death | Time to event | TQIP chart review | During Hospitalisation |
| Patient 6-month mortality | Binary; 1/0 | TQIP chart review | 6 months | |
| Withdrawal of life- supporting treatment | Time between admission and withdrawal of life-supporting treatment at the EOL | Time to event | TQIP chart review | During hospitalisation |
| Practitioner opinion survey | Trauma surgeon’s impressions of the communication tool (12 items) | Ordinal; 5-point Likert scale | Surgeon | On studyCompletion |
T0= 3 months before implementation, T1: 1 year after T0.
ICUintensive care unit TQIPTrauma Quality Improvement Program
Planned analysis
Sample size calculation
Based on our primary hypothesis that family members in the intervention arm will be more likely to receive higher quality communication, we estimate the need for 1500 family-reported QOC surveys (750/group) to detect a difference of 0.40 in QOC scores. This detectable difference is consistent with other interventions designed to effectively improve serious illness communication and smaller differences are unlikely to be considered meaningful by clinicians, patients and families, and researchers. Our calculation assumes eight study sites, a two-sided type 1 error rate of 0.05, an SD of 1.92 and an intraclass correlation coefficient (ICC) of 0.001 based on preliminary data.33 Based on these assumptions, we will have 80% power to detect a significant mean difference of 0.40. If we consider the upper limit of the 95% CI for the ICC, that is, ICC=0.02, the detectable difference increases to 0.48.
Based on 2019 historical TQIP data, we anticipate following approximately 4500 patients. We estimate enrolling up to 1600 clinicians.
Primary outcomes analysis
Using an intention-to-treat analysis, we will use a linear mixed-effects model to test the effect of the tool on family-reported QOC as compared with usual care. The model will include a treatment indicator variable, a fixed effect for time (measured categorically by wave) and a random intercept for the site.34
Key secondary outcomes analysis
We will examine the effect of the intervention on key secondary outcomes in the context of linear mixed-effects models. Models examining clinician outcomes, that is, MMD-HP and MBI scores, will include a poststudy indicator variable and a random intercept for the site. For patient health outcomes, specifically ICU LOS, we will include a treatment indicator variable, as well as fixed effects for the time, patient comorbidity and injury severity and a random intercept for the site.
Exploratory analysis
Given the intricacies of examining ICU LOS when follow-up may be truncated due to patient death,35 we will perform two exploratory analyses. First, we will examine ICU LOS among decedents only (ie, those who died during their ICU hospitalisation) using a linear mixed-effects model. Second, we will implement causal mediation analysis to determine if the effect that Best Case/Worst Case intervention has on ICU LOS is mediated by in-hospital mortality.
Missing data
Following the principles of the National Research Council report, we designed this study to minimise the potential for missing data.36 We expect missing outcomes will be minimal for QOC as it is collected in person or via telephone at the time of family member enrolment. We will handle missing data due to item non-response in a manner consistent with QOC scoring guidelines, that is, we will impute unanswered survey questions with the respondent’s median score for all answered questions within the subsection (general QOC or EOL QOC) if at least half the questions were answered.26 We will require respondents to have subscale scores to receive an overall QOC score. We anticipate low rates of missing data for patient outcomes related to their trauma admission since all participating trauma centres report this information to the TQIP registry for quality assurance purposes.
Patient and public involvement
Patients and members of the public were not involved in the design or conduct of the study. There is planned engagement of patients and family stakeholders via the Coalition for National Trauma Research and the Injury Research Engagement Panel for reporting and dissemination of this research.
Ethics and dissemination
Ethical review
This study presents minimal risks to participants. Following an approach well described in health services research,37 38 we will implement a quality improvement initiative within an interventional research study. Implementing the Best Case/Worst Case-ICU communication tool is considered a quality improvement initiative because it aims to improve guideline-recommended standard practice for discussing care with patients and families. Our systematic investigation of the effect of the Best Case/Worst Case-ICU communication tool on clinician and family member experiences and patient outcomes aligns with the federal definition of research.39 We will not obtain patient consent for randomisation to the treatment group or delivery of the intervention because the intervention qualifies as quality improvement, compares guideline recommended care to usual care and implementation occurs at the study-site level. Additionally, we will not obtain patient consent for clinical data collection as the TQIP quality registry collects all patient data regardless of study participation. We will not obtain consent for clinician training as the tool is an educational initiative to support both clinicians and patients in having high-quality conversations. We will obtain verbal consent for study procedures, specifically, family member and clinician surveys at the time of survey completion and participants may withdraw at any time (onlinesupplemental materials 1 2). Study participation will not affect the care a patient receives or clinicians’ professional standing. Institutional review board (IRB) approval was granted at the University of Wisconsin, and study sites ceded review to the primary IRB. An independent data safety monitoring board (DSMB), representing a variety of backgrounds, including biostatistics and trauma care, will serve as the data and safety advisory group for all study sites. The DSMB met prior to study initiation and will meet again after 12 months of family-member data collection and at the end of data collection. We will submit all reportable events to the DSMB and the primary IRB in accordance with their reporting guidelines. As this is a minimal-risk study, there are no predefined stopping points due to futility, efficacy or harms.
Relevance and dissemination
Our intervention uses scenario planning to disrupt the clinical momentum that promotes passive accumulation and escalation of life-supporting treatments without active consideration of whether these treatments and their associated outcomes are consistent with the patient’s overall health goals and prognosis. If shown to be effective, our intervention could support improved patient-centred outcomes for families, clinicians and patients with serious illness in the ICU and reduce strain on ICU resources. We plan to publish study results in peer-reviewed journals. Information about the intervention, including training materials, is available at https://patientpreferences.org/bcwc-icu/. A deidentified data set comprised survey data, metadata and analytical code will be made available through the National Archive of Computerized Data on Aging or a comparable NIH-supported repository. Patient-level data collected by the TQIP registry are available on request from the ACS, who administer the TQIP programme. Evidence of the effectiveness of the Best Case/Worst Case-ICU communication tool would support investment in clinician communication training, wide adoption by trauma centres and provide new knowledge about how scenario planning can assist decision-makers during serious illness.
supplementary material
Acknowledgements
We would like to thank the ACS and the Coalition for National Trauma Research (CNTR) for their collaboration. Specifically, we would like to acknowledge the support of Bhavin Patel (ACS) and Dr Michelle Price (CNTR). We would also like to thank the Research Coordinators for their dedication.
Footnotes
Funding: This work was supported by the National Institutes of Health (grant number R01AG078242).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-083603).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Lily Stalter, Email: stalter@surgery.wisc.edu.
Bret M Hanlon, Email: bmhanlon@wisc.edu.
Kyle J Bushaw, Email: bushaw@surgery.wisc.edu.
Kristine L Kwekkeboom, Email: kwekkeboom@wisc.edu.
Amy Zelenski, Email: zelenski@medicine.wisc.edu.
Melanie Fritz, Email: mfritz@uwhealth.org.
Anne Buffington, Email: buffington@surgery.wisc.edu.
Deborah M Stein, Email: dstein@som.umaryland.edu.
Christine S Cocanour, Email: cscocanour@ucdavis.edu.
Anamaria J Robles, Email: arobles@ucdavis.edu.
Jan Jansen, Email: jjansen@uabmc.edu.
Karen Brasel, Email: brasel@ohsu.edu.
Kathleen M O'Connell, Email: katmo@uw.edu.
Mark D Cipolle, Email: mark.cipolle@lvhn.org.
Patricia Ayoung-Chee, Email: payoungchee@msm.edu.
Rachel Morris, Email: ramorris@mcw.edu.
Rondi B Gelbard, Email: rgelbard@uabmc.edu.
Rosemary A Kozar, Email: rkozar@som.umaryland.edu.
Stephanie Lueckel, Email: stephanie_lueckel@brown.edu.
Margaret Schwarze, Email: schwarze@surgery.wisc.edu.
References
- 1.Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54:1590–5. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control 10 leading causes of nonfatal injuries, United States, 2014, all races, both sexes, disposition: All cases, ages: 65-85. 2014. https://www.cdc.gov/injury/wisqars/index.html Available.
- 3.Fleischman RJ, Adams AL, Hedges JR, et al. The optimum follow-up period for assessing mortality outcomes in injured older adults. J Am Geriatr Soc. 2010;58:1843–9. doi: 10.1111/j.1532-5415.2010.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried TR. Shared decision making-finding the sweet spot. N Engl J Med. 2016;374:104–6. doi: 10.1056/NEJMp1510020. [DOI] [PubMed] [Google Scholar]
- 5.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US medicare population. Med Care. 2007;45:386–93. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field MJ, Cassel CK. Approaching death: Improving care at the end of life. 1997. [PubMed]
- 7.Fassier T, Azoulay E. Conflicts and communication gaps in the intensive care unit. Curr Opin Crit Care. 2010;16:654–65. doi: 10.1097/MCC.0b013e32834044f0. [DOI] [PubMed] [Google Scholar]
- 8.Danjoux Meth N, Lawless B, Hawryluck L. Conflicts in the ICU: perspectives of administrators and clinicians. Intensive Care Med. 2009;35:2068–77. doi: 10.1007/s00134-009-1639-5. [DOI] [PubMed] [Google Scholar]
- 9.Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: Estimated costs and potential for savings. JAMA. 2019;322:1501–9. doi: 10.1001/jama.2019.13978. [DOI] [PubMed] [Google Scholar]
- 10.Mosenthal AC, Murphy PA. Trauma care and palliative care: time to integrate the two? J Am Coll Surg. 2003;197:509–16. doi: 10.1016/S1072-7515(03)00651-3. [DOI] [PubMed] [Google Scholar]
- 11.Neuman MD, Bosk CL. What we talk about when we talk about risk: refining surgery’s hazards in medical thought. Milbank Q. 2012;90:135–59. doi: 10.1111/j.1468-0009.2011.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarze ML, Taylor LJ. Managing uncertainty - Harnessing the power of scenario planning. N Engl J Med. 2017;377:206–8. doi: 10.1056/NEJMp1704149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakin JR, Jacobsen J. Softening our approach to discussing prognosis. JAMA Intern Med. 2019;179:5–6. doi: 10.1001/jamainternmed.2018.5786. [DOI] [PubMed] [Google Scholar]
- 14.Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391. doi: 10.1136/bmj.h391. [DOI] [PubMed] [Google Scholar]
- 15.Sudore RL, Landefeld CS, Williams BA, et al. Use of a modified informed consent process among vulnerable patients: a descriptive study. J Gen Intern Med. 2006;21:867–73. doi: 10.1111/j.1525-1497.2006.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Surgeons National trauma data standard data dictionary 2023 admissions. Chicago, IL. 2023.
- 17.Monaghan TF, Agudelo CW, Rahman SN, et al. Blinding in clinical trials: Seeing the big picture. Medicina (Kaunas) 2021;57:647. doi: 10.3390/medicina57070647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, et al. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. 2014;43:1272–83. doi: 10.1093/ije/dyu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kon AA, Davidson JE, Morrison W, et al. Shared decision-making in intensive care units. Executive summary of the American College of critical care medicine and American thoracic society policy statement. Am J Respir Crit Care Med. 2016;193:1334–6. doi: 10.1164/rccm.201602-0269ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truog RD, Campbell ML, Curtis JR, et al. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med. 2008;36:953–63. doi: 10.1097/CCM.0B013E3181659096. [DOI] [PubMed] [Google Scholar]
- 21.Kruser JM, Cox CE, Schwarze ML. Clinical momentum in the intensive care unit. A latent contributor to unwanted care. Ann Am Thorac Soc. 2017;14:426–31. doi: 10.1513/AnnalsATS.201611-931OI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruser JM, Benjamin BT, Gordon EJ, et al. Patient and family engagement during treatment decisions in an ICU: A discourse analysis of the electronic health record. Crit Care Med. 2019;47:784–91. doi: 10.1097/CCM.0000000000003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Needle JS, Liaschenko J, Peden-McAlpine C, et al. Stopping the momentum of clinical cascades in the PICU: Intentional responses to the limits of medicine. J Palliat Care. 2021;36:12–6. doi: 10.1177/0825859719851487. [DOI] [PubMed] [Google Scholar]
- 24.Kruser JM, Pecanac KE, Brasel KJ, et al. “And I think that we can fix it”: mental models used in high-risk surgical decision making. Ann Surg. 2015;261:678–84. doi: 10.1097/SLA.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuman MD. Surgeons’ decisions and the financial and human costs of medical care. N Engl J Med. 2010;363:2382–3. doi: 10.1056/NEJMp1009621. [DOI] [PubMed] [Google Scholar]
- 26.Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. J Palliat Med. 2006;9:1086–98. doi: 10.1089/jpm.2006.9.1086. [DOI] [PubMed] [Google Scholar]
- 27.Modes ME, Heckbert SR, Engelberg RA, et al. Patient-reported receipt of goal-concordant care among seriously Ill outpatients—prevalence and associated factors. J Pain Symptom Manage. 2020;60:765–73. doi: 10.1016/j.jpainsymman.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein EG, Whitehead PB, Prompahakul C, et al. Enhancing understanding of moral distress: The measure of moral distress for health care professionals. AJOB Empir Bioeth. 2019;10:113–24. doi: 10.1080/23294515.2019.1586008. [DOI] [PubMed] [Google Scholar]
- 29.Maslach C, Jackson SE, Leiter MP. Maslach burnout inventory: Scarecrow Education. 1997.
- 30.O’Connor A, Cranney A. User manual - acceptability (document on the Internet) 1996. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Acceptability.pdf Available.
- 31.Dyrbye LN, Meyers D, Ripp J, et al. A pragmatic approach for organizations to measure health care professional well-being. NAM Perspectives; 2018. [Google Scholar]
- 32.West CP, Dyrbye LN, Sloan JA, et al. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24:1318–21. doi: 10.1007/s11606-009-1129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann CJ, Zelenski AB, Buffington A, et al. Best case/worst case for the trauma ICU: Development and pilot testing of a communication tool for older adults with traumatic injury. J Trauma Acute Care Surg. 2021;91:542–51. doi: 10.1097/TA.0000000000003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28:182–91. doi: 10.1016/j.cct.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Harhay MO, Ratcliffe SJ, Small DS, et al. Measuring and analyzing length of stay in critical care trials. Med Care. 2019;57:e53–9. doi: 10.1097/MLR.0000000000001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Research Council The prevention and treatment of missing data in clinical trials. 2010. [PubMed]
- 37.White DB, Angus DC, Shields A-M, et al. A randomized trial of a family-support intervention in intensive care units. N Engl J Med. 2018;378:2365–75. doi: 10.1056/NEJMoa1802637. [DOI] [PubMed] [Google Scholar]
- 38.Lincoln T, Shields A-M, Buddadhumaruk P, et al. Protocol for a randomised trial of an interprofessional team-delivered intervention to support surrogate decision-makers in ICUs. BMJ Open. 2020;10:e033521. doi: 10.1136/bmjopen-2019-033521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Department of Health and 2018 Requirements (2018 common rule) 2018. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/revised-common-rule-regulatory-text/index.html#46.102 Available.